Table of Contents
ToggleChemical kinetics is the branch of chemistry that deals with the rate and the mechanism of chemical reactions. It studies how fast a chemical reaction proceeds as well the how does it proceeds (mechanism).
Rate of chemical reaction
The rate of a chemical reaction can be defined as the change in concentration of reactant per unit time. It is also called reaction velocity. During a chemical reaction, the concentration of reactants goes on decreasing and the concentration of products increases with time.
Let us consider a chemical reaction, A + B → C + D
The rate of the above reaction is represented by dx/dt. where dx represents the amount of reactant changed in time dt.
The rate in terms of Reactant:
Rate= -d[A]/dt=-d[B]/dt.
Here -ve sign indicates that the concentration of reactant decreases with time.
Similarly, Rate in terms of Product:
Rate= +d[C]/dt= +d[D]/dt.
Here, +ve sign indicates that the concentration of products increases with time.
Let’s consider another reaction with stoichiometric coefficients a,b,c and d.
aA + bB → cC + dD
here, the rate of reaction is represented as:
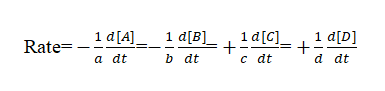
Instantaneous rate of reaction
Instantaneous rate of a chemical reaction can be defined as the change in concentration of the reactant in an infinitesimally small interval of time.
Unit of rate of chemical reaction
The unit of rate or reaction velocity is mole per liter per unit time or molL-1 time-1. The time may be second, minute or hours.
Rate constant of Chemical Reaction
Let us consider a chemical reaction, A + B → C + D
Let [A] and [B] are the concentration of reactant A and B at time t respectively then according to the law of mass action,
rate ∝ [A][B]
dx/dt= k [A][B]
where k is a constant called rate constant or specific reaction rate.
When [A]=[B]=1, then dx/dt= k .
Therefore, the Rate constant can be defined as the rate of reaction when the concentration of the reactant is unity.
Types of chemical Reaction
On the basis of the rate of chemical reaction, chemical reactions are of the following types:
- Fast reaction
- Moderate reaction
- Slow reaction
Fast reaction: Those chemical reactions which have a very high rate of reaction are called fast reactions. The rate of fast reaction can not be measured by the general method but it needs special techniques. Example: Ionic reaction is an example of a fast reaction.
HCl+NaOH→ NaCl+ H2O
Moderate reaction: Those chemical reactions that have an average rate of reaction are called moderate reactions. The rate of moderate reaction can be measured by using conventional methods. Example: Decomposition of H2O2
2H2O2→ 2H2O +O2
Slow reaction: Those chemical reactions that have a very low rate of reaction are called moderate reactions. These slow reactions may take a few days to months to complete. Example: Rusting of iron is an example
Fe2O3+xH2O→ Fe2O3.xH2O (rust)
Factors affecting rate of reaction
Factors that affect the rate of a chemical reaction are listed below:
- Nature of reactant
- Concentration
- Temperature
- Catalyst
- Presence of sunlight
- Pressure
1. Nature of reactant:
The state of the reactant also affects the rate of a chemical reaction. If the reactants are in the gaseous state, the rate of reaction is considerably higher than the reactant in a liquid state and solid-state. This is because gaseous reactant molecules are free to move and collide with each other to give the product at a fast rate.
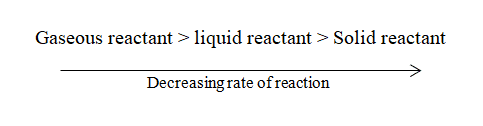
Similarly, the size of the particle of reactants plays a significant role in the rate of reaction. On decreasing the particle size of the reactant, the surface area of the reactant increases, and hence effective collision also increases which leads to an increase in the rate of the chemical reaction. For example: when solid calcium carbonate is reacted with HCl, the reaction proceeds with a significant rate but when powdered calcium carbonate is treated with HCl, the rate increases significantly and the reaction proceed vigorously.
CaCO3 + 2HCl→ CaCl2 + H2O + CO2
Further, the reactions involving polar and ionic reactants are faster in comparison to other reactions involving the rearrangement of bonds or electrons.
2. Concentration of reactant:
The rate of reaction is directly proportional to the concentration of reactants. On increasing the concentration of reactant, the rate also increases. Therefore, the rate of reaction is decreased when the reactant concentration decreases.
We can take the example of the reaction between calcium carbonate and HCl. The rate of reaction is greater when the concentrated HCl is used instead of dilute HCl.
3. Temperature
The rate of reaction increases by increasing the temperature of the reaction system. This is because, on increasing temperature, the kinetic energy of the reactant molecule increases, and the molecule having sufficient energy to cross the energy barrier also increases, hence the rate of reaction increases.
The rate of a reaction becomes almost double or tripled for every Co 10 rise in temperature. The temperature coefficient of a reaction is defined as the ratio of rate constants at two different temperatures differing by 10 °C.
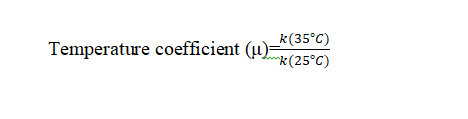
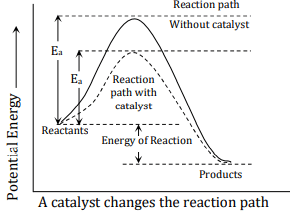
4. Catalyst
The catalyst increases the rate of a chemical reaction by either lowering the activation energy or changing the path of the reaction. More decrease in the activation energy of reaction higher will be the reaction rate.
5. Presence of Sunlight
Some chemical reactions are influenced by electromagnetic radiation, especially UV and visible light. Such reactions are called photochemical reactions. The light provides sufficient energy to the molecules to reach the activation energy.
Example: Reaction between H2 and Cl2 in presence of light.
H2 + Cl2 → 2HCl
6. Pressure
The effect of pressure is considered in the case of gaseous reactants. If the pressure of the reactant system is increased, the gaseous reactant comes very close to each other and there is a chance of collision between the reactant molecule. Therefore, on increasing the pressure of the gaseous reactant, the rate of reaction increases.
Molecularity of reaction
Molecularity of a chemical reaction is defined as the total number of reactant molecules involved in a balanced chemical equation. It may be a unimolecular, bimolecular reaction on the basis of a number of reactant molecules.
Example: Inversion of cane sugar is a bimolecular reaction.
C12H22011 + H20 ⇌ C6H1206 + C6H1206
- Reactions having molecularity one, two, three, etc. are known as unimolecular, bimolecular and trimolecular reactions, respectively.
- Molecularity can not be greater than three because more than three molecules may not mutually collide with each other.
- Molecularity of a reaction can’t be zero, negative or fractional.
Order of chemical reaction
Order of reaction is defined as the sum of the power of each concentration term of reactants in the rate law expression. it can also be defined as the total number of reactant molecules in rate-determining step.
Consider a reaction, A+B→ Product
The rate law expression is, Rate=k[A]1[B]1
Order= 1+1= 2
Similarly, for a reaction xA+yB → Product
Rate= k[A]x[B]y
Therefore, order=x+y
- Order of a reaction may be zero, negative, positive or in fraction and greater than three.
On the basis of the order of reaction, the following types of reactions are reported.
- zero order reaction
- first order reaction
- second order reaction
- third order reaction
These are discussed in the next content in detail.
Chemical kinetics video
FAQs:
What is rate of a reaction?
The rate of a chemical reaction can be defined as the change in concentration of reactant per unit time. It is also called reaction velocity.
What do you mean by the instantaneous rate of reaction?
Instantaneous rate of a chemical reaction can be defined as the change in concentration of the reactant in an infinitesimally small interval of time
What is the unit of rate of reaction?
The unit of rate or reaction velocity is mole per liter per unit time or molL-1 time-1
What is the molecularity of a chemical reaction?
Molecularity of a chemical reaction is defined as the total number of reactant molecules involved in a balanced chemical equation.
What do you mean by order of a reaction?
Order of reaction is defined as the sum of the power of each concentration term of reactants in the rate law expression. it can also be defined as the total number of reactant molecules in the rate-determining step






