Table of Contents
ToggleThe total heat content of a system at constant pressure is equivalent to the internal energy (E) plus the PV energy. This is called the enthalpy of the system and is represented by the symbol ‘H’. Thus, enthalpy is defined by the equation:
H = E + PV
where P and V are the pressure and volume of the system, respectively. If H1 and H2 are the enthalpies of the system in the initial and final states,
H1 = E1 + P1V1
H2 = E2 + P2V2
H2 – H1 = (E2 – E1) + (P2V2 – P1V1)
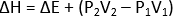
When the pressure remains constant throughout the process, then
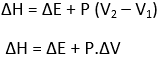
The change in enthalpy at constant pressure is equal to the increase in internal energy plus any pressure-volume work done. Hence, at constant pressure, ΔH represents the heat absorbed by a system in going from an initial state to a final state provided the only work done is PV- work.
According to the first law of thermodynamics
ΔE = q-W
since at constant pressure
W = P. ΔV
ΔE + P. ΔV =qp
Hence, an increase in enthalpy equals the heat absorbed at constant pressure when no work other than P.ΔV work is done.
The enthalpy change, ΔH is positive if H2 > H1 and the process or reaction will be endothermic whereas ΔH is negative if H1 > H2 and the reaction will be exothermic.
Enthalpy of Formation
The enthalpy of formation of a compound is defined as the change in enthalpy of the system when one mole of the compound is formed from its elements. It is denoted by ΔHf. for example;
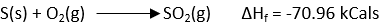
The standard enthalpies of formation of a compound are defined as the change in enthalpy of the system when one mole of a compound is formed from its element in its standard states.
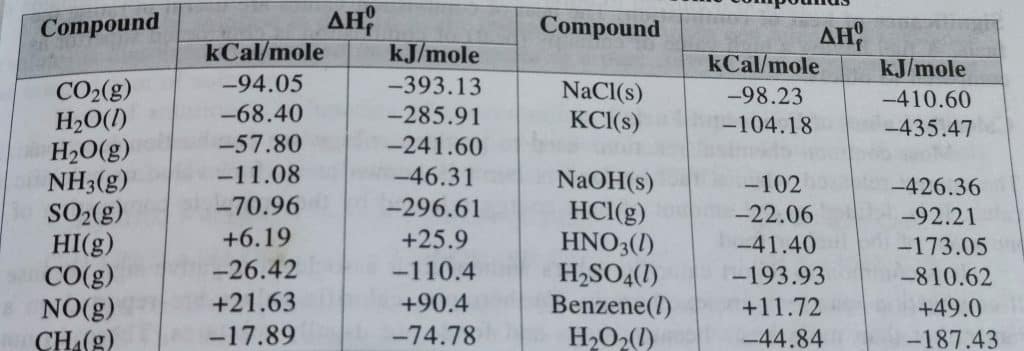
Standard enthalpy of formation of some compounds
Enthalpy of Neutralization
The enthalpy of neutralization of an acid at a given temperature is defined as the change in enthalpy (ΔH) accompanying the neutralization of one gram equivalent of the acid by a base or vice-versa in dilute solution at that temperature.
Heat of neutralization in some cases is given below.

Enthalpy of Vaporization
It is defined as the heat change or enthalpy change when one mole of a liquid is converted into vapors or a gaseous state at its boiling point. For example, When one mole of water is converted into steam at 1000C or 373 K, the heat absorbed is 9.71kCal, which is the heat of vaporization of water. The change can be represented as
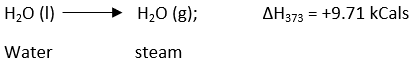
FAQs
What is enthalpy?
The total heat content of a system constant pressure is equivalent to the internal energy (E) plus the PV energy. This is called enthalpy of the system and is
How to calculate enthalpy change?
The mass of your reactants, s, the specific heat of your product, and ∆T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction using the formula ∆H = m x s x ∆T.






