Table of Contents
ToggleWhat is a chemical reaction? Simply chemical reaction may sound a bit of a scientific term but every simple reaction occurring around the world can be accounted for in this terminology. In general word, a Chemical reaction is a process that leads to a chemical change in the reactants. It is responsible for the rearrangement of the constituent atoms of the reactants and forms different substances as a product.
Chemical reactions occur when chemical bonds between atoms are formed or broken. The substances that undergo a chemical reaction are known as reactants, while the substances formed as a result of the reaction are known as products. Chemical reactions can often be represented by a chemical equation and include changes that involve the locations of electrons in the formation and breakage of chemical bonds between atoms. Simple contact, contact of the solution, Heat, light, electricity, catalyst, pressure, etc are some of the methods of carrying out a chemical reaction.
Chemical changes and Physical changes are two different phenomena. Generally, physical changes are the changes in states, and if occur, only the properties of the substance will change but the chemical identity will remain the same. However, a chemical change causing a chemical reaction is usually observed with physical changes such as color change, precipitation, heat production, and so on.
Chemical reactions are an integral part of our surroundings from the reaction in sun to the metabolism of food in our body. Rusting of iron, burning fuels, brewing beer, fermentation of wine, photosynthesis, smelting of iron, etc. are some of the examples of chemical reactions.
What is a Chemical Reaction – Definition
Chemical reaction is a process in which the bonds in reacting molecules (reactants) are broken and new bonds leading to the production of product molecules are formed. It is indicated by an arrow describing the direction of the chemical reaction. The arrow may be either (→) indicating an irreversible reaction that runs in only one direction or (⇆/↔) indicating a reversible reaction that runs in both directions. The rate of a chemical reaction is affected by the factors like temperature, pressure, and concentration of the reactants.
Types of Chemical reaction
Chemical reactions are mainly classified into the following types:
1. Synthesis or Combination reaction
The process of chemical change by which a compound is formed by the direct combination of two or more than two reacting species is known as synthesis or combination reaction. This combination may be brought about by the application of heat, electricity, and pressure. Some of the examples are:
Burning of magnesium ribbon in oxygen to form magnesium oxide

Combination of H2 and Cl2 in presence of sunlight results in the formation of HCl.
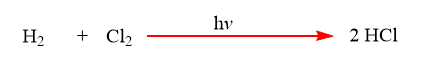
2. Analysis or Decomposition reaction
The process of breaking up of a compound into two or more than two constituents by the application of heat, electricity, or light is known as decomposition. It is just the opposite of the synthesis or combination. The examples involving this reaction are:
Thermal decomposition of calcium carbonate gives calcium oxide and carbon dioxide.

Decomposition of water into hydrogen and oxygen in the presence of current electricity.

3. Replacement or Displacement reaction
Displacement reaction is the process by which one component of a chemical is ejected and replaced by another component. For example,
Zinc reacts with hydrochloric acid to displace hydrogen leading to the formation of ZnCl2

4. Double displacement reaction
It is the process by which two compounds participating in a reaction that forms two new compounds exchange their radicals with one another. This process is called a double displacement reaction. For example:
Action of barium chloride solution on sulphuric acid gives barium sulphate precipitate and HCl.

5. Neutralization (Acid-Base reaction)
The reaction in which an acid and base combine to give salt and water is called neutralization or acid-base reactions. Some of the examples are:

6. Hydrolysis reaction
A chemical reaction that is brought with the aid of water is said to be hydrolysis. For example:

7. Rearrangement reaction
Rearrangement reaction refers to the process of creating a new compound as a result of internal atom position changes inside an existing compound’s molecular formula without altering the individual properties of each constituent.

8. Polymerization reaction
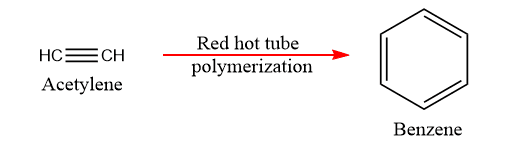
Polymerization is the method by which two or more simple molecules are combined to form a single complex one. For example:
When acetylene gas is passed through a red hot copper tube, three molecules of it combine to form one molecule of benzene.
Methods of Bringing about a chemical reaction
The chemical reaction that takes place in nature is governed by several conditions that vary from reaction to reaction. A chemical reaction can be induced by various methods. Some of the methods of bringing a chemical reaction are discussed below:
1. Simple contact: Some compounds undergo chemical reactions when they are brought together into contact with one another.

2. Contact of solution: Certain chemical reactions do not take place readily when reactants are brought in contact in the solid state but reaction occurs readily when contact is made between their solutions.

3. Light: Some chemical reactions take place only under the influence of light.
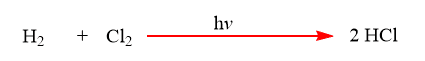
4. Heat: Some reactions take place when the reactants are heated as heat increases the speed of a chemical reaction.
5. Catalyst: The substance which changes the speed of a chemical reaction without itself undergoing any chemical change at the end of the reaction, is called a catalyst. This phenomenon of changing the rate of reaction is called catalysis. Enzymes are biological catalysts and are very useful to catalyze most biochemical reactions.
6. Pressure: Many chemical reactions take place by the application of pressure on the reactants. Usually, gaseous reactants are carried out under pressure. The speed of gas increases by increasing the pressure.
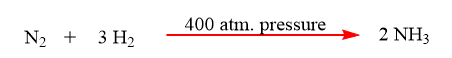
7. Electricity: Both the combination and decomposition processes can be achieved via the use of electricity.
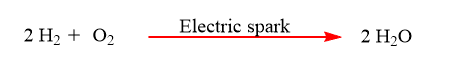
Chemical reaction video
FAQs
What subatomic particles are responsible for all chemical reactions?
Electrons are responsible for the breakage and formation of chemical bonds resulting in chemical reactions.
How do enzymes speed up chemical reactions?
Enzymes are the biocatalyst that speeds up reactions by lowering the activation energy required to initiate a reaction.






