Table of Contents
ToggleWhat is two-step synthesis?
A two-step synthesis is a synthetic approach or pathway through which a more complex compound is built up in steps from the initial starting material. In this approach, the product of the first reaction becomes the starting material for the next, and so forth. The preparation of phthalimide from phthalic acid and, the preparation of p-aminobenzene from aniline are examples of two-step synthesis.
Preparation of Phthalimide from Phthalic acid
Phthalimide can be synthesized by a two-step synthesis starting with phthalic acid, as follows:
Step 1: Preparation of phthalic anhydride from phthalic acid
On strong heating of phthalic acid to about 200 °C, white-colored needle-shaped crystals of phthalic anhydride are formed.
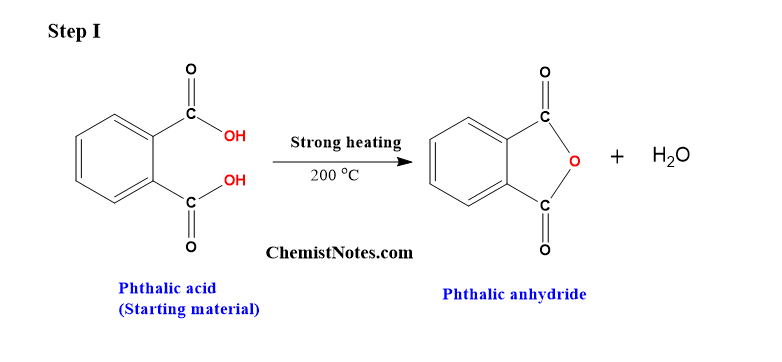
This is the dehydration step in which water molecules are removed.
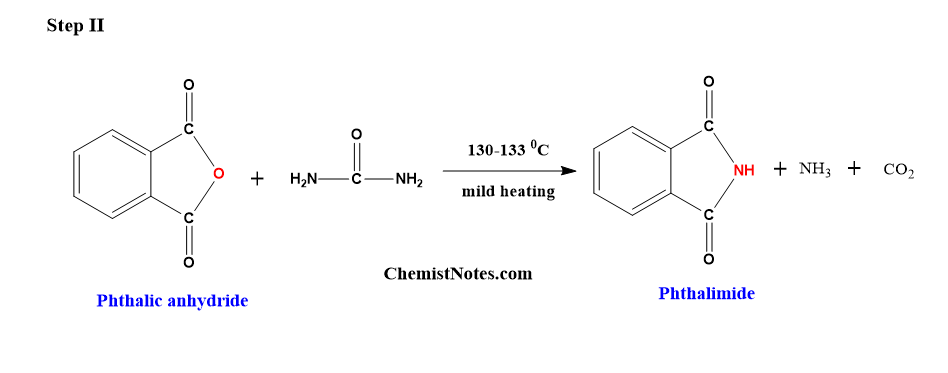
Step 2: Preparation of phthlimide from phthalic anhydride
When a mixture of phthalic anhydride and urea is heated to about 130–133 °C in a sand bath, after frothing the mass, a spongy solid called phthalimide is obtained.
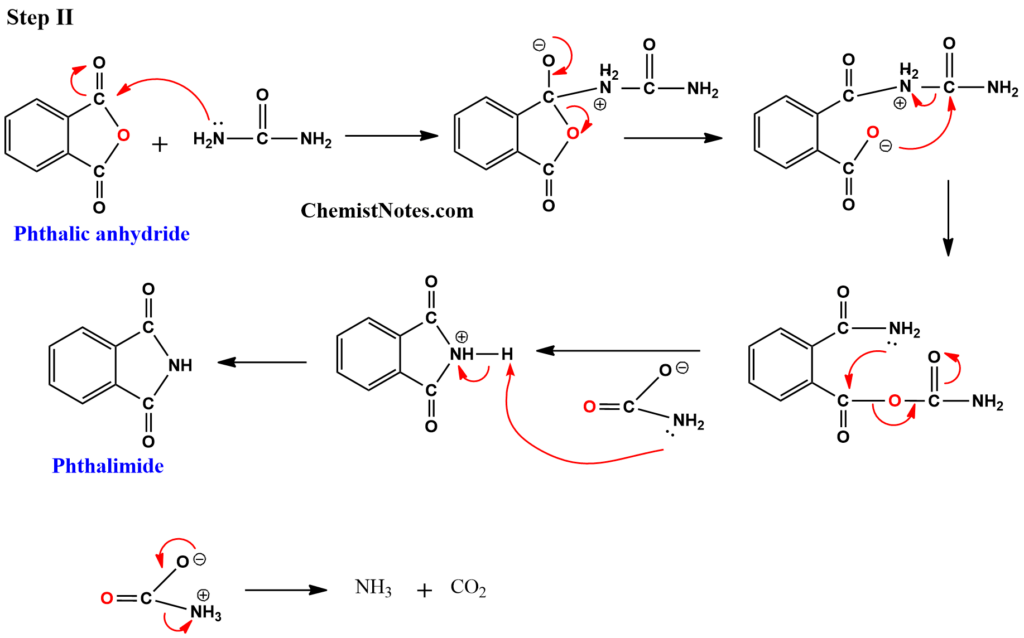
Mechanisms involved in the conversion of phthalic acid to phthalimide
Let’s see how phthalic acid is converted to phthalimide mechanistically. In the first step, phthalic acid is converted to phthalic anhydride.
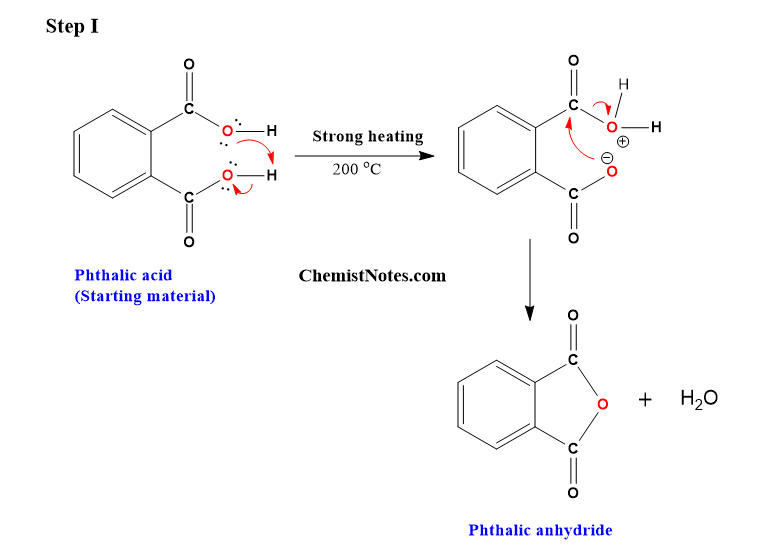
In the second step, one of the carbonyl carbons of phthalic anhydride is attacked by the lone pairs of electrons of nitrogen atoms in urea.
Procedure
- Take 1 gram of phthalic acid and pour it into a clean and dry conical flask.
- Then, heat the conical flask containing phthalic acid strongly to about 200 °C in the sand bath by covering it with a funnel for an hour.
- After the complete formation of needle-shaped crystals of phthalic anhydride on the inner surface of the flask and funnel, collect the crystals in a watch glass. Then, calculate the percentage yield.
- Transfer the formed phthalic acid into a clean and dry round bottom flask.
- Add 0.256 grams of Urea to the round bottom flask and mix homogeneously.
- Heat the mixture slowly in a sand bath maintaining the temperature between 130-135 °C. Stop heating when frothing occurs.
- Cool the flask and pour 20–25 mL of water into the cooled round bottom flask.
- Filter spongy solids and recrystallize with methanol.
- Calculate yield and yield percentage
- Calculate the melting point and submit your sample for IR spectroscopy.
Observation
Needle-shaped white crystals of phthalic anhydride will appear on the inner surface of the flask and funnel. Later, after the addition of urea and heating, you will observe a spongy solid of phthalimide.
Calculation
Mass of phthalic acid taken = 1 g
Mass of phthalic anhydride obtained= 0.752 g
No. of moles of phthalic acid = (Given weight/Molecular weight)= 1/16= 0.0060 moles
No. of moles Phthalic anhydride = given weight/ molecular weight = x/148
We have,
1 mole of phthalic acid gives 1 mole of phthalic anhydride
0.0060= x/148
x= 148 × 0.0060 = 0.888 gram
so, % yield= (actual yield/theoretical yield) × 100% = 84.6%
Calculation of % yield for Phthalimide:
Mass of phthalic anhydride taken= 0.752 g
Mass of phthalimide obtained = 0.482 g
No. of moles of Phthalic anhydride= given mass/ molecular weight = 0.752/148= 0.0051
No. of moles of phthalimide= x/147
We know,
1 mole of phthalic anhydride gives x moles of phthalimide.
or, 0.0051= x/147
or, x= 0.7497 g
or, % yield= (actual yield/theoretical yield) × 100% = (0.482/0.7497) × 100% = 64.29%
Melting point of Phthalic anhydride and phthalimide
The melting points of phthalic anhydride and phthalimide can be determined by using Thiele’s tube. The reported melting points and observed melting points are listed below in the table.
| Compounds | Reported melting points | Observed melting points |
| Phthalic anhydride | 131 °C | 125 °C |
| Phthalimide | 238 °C | 234 °C |
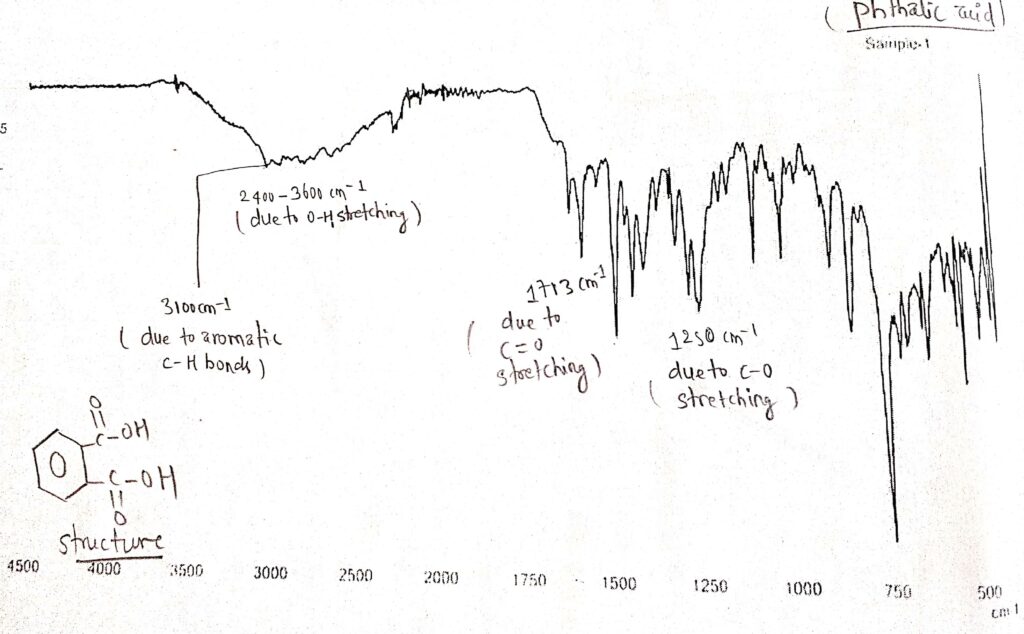
IR spectrum of Phthalic acid
From the spectrum of Phthalic acid, four types of stretching were observed, such as C=O stretching, C-O stretching, O-H stretching, and C-H stretching. There is a broad peak at about 2500–3600 cm-1 due to O-H stretching. Also, a sharp peak at around 1713 cm-1 was observed, which is due to C=O stretching. Moreover, a sharp peak at about 1250 cm-1 was observed, which is due to C-O stretching. Due to aromatic C-H stretching, a sharp peak at about 3100 cm-1 was observed.
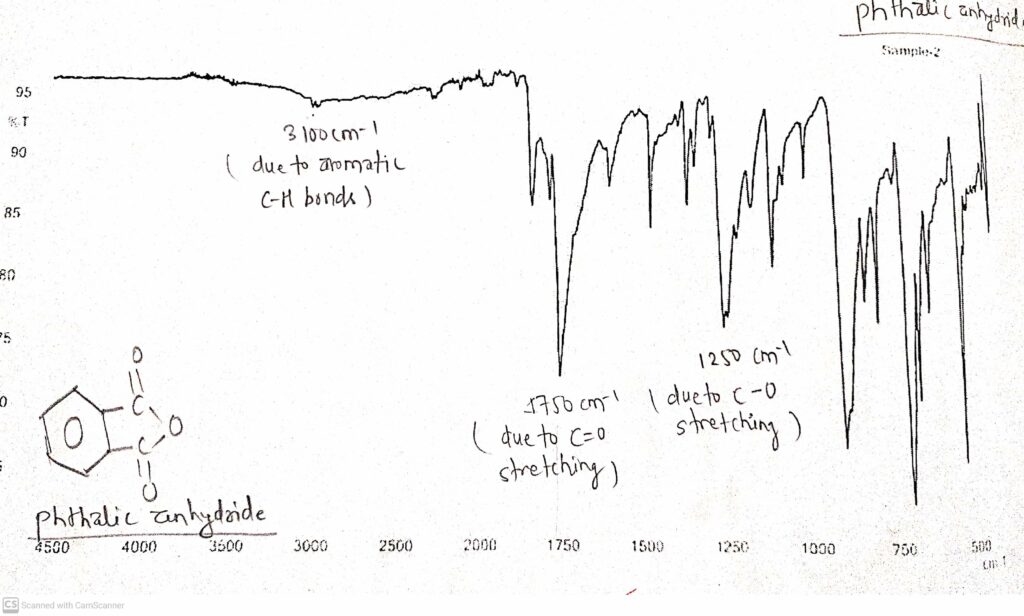
IR spectrum of Phthalic anhydride
In the IR spectrum of phthalic anhydride, there are important stretching namely, C=O stretching, C-O stretching and C-H stretching. C=O stretching was observed at about 1750 cm-1. C-O stretching peak was observed at about 1250 cm-1. Due to the aromatic C-H bond, a peak at around 3100 cm-1 was observed.
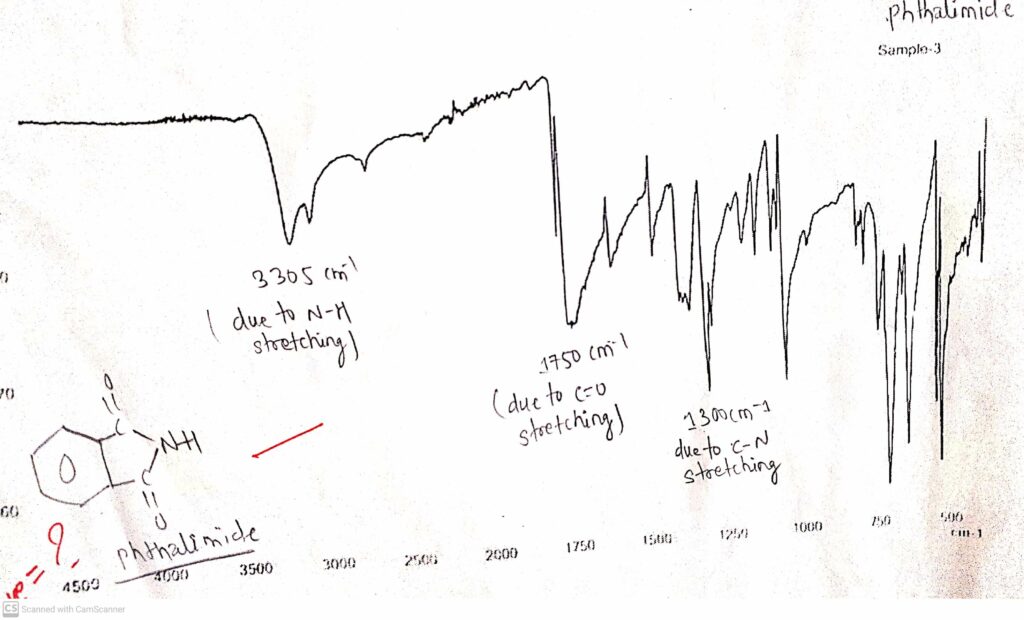
IR spectrum of Phthalimide
In the IR spectrum of phthalimide, two fang-like structures were observed at around 3200 cm-1 which is due to N-H stretching. Another sharp sword-like peak was observed around 1750 cm-1 which is due to C=O stretching. Another peak was observed around 1300 cm-1 which is due to the C-N bond.
Result
Needle-shaped crystals of phthalic anhydride and a spongy solid mass of phthalimide were obtained. The IR spectra of phthalic acid, phthalic anhydride, and phthalimide were obtained and analyzed.
Conclusion
Hence, phthalimide can be prepared from phthalic acid by two-step synthesis, and the functional groups of the starting and synthesized compounds can be identified by IR spectroscopy.