Table of Contents
ToggleThe polymerization which initiates by the formation of cation or positively charged species is called cationic polymerization. The active site of cationic polymerization is either carbonium ions or oxonium ions. Generally, the monomer of these polymerizations includes electron-donating substituents, in which the polarization of the double bond makes them sensitive to electrophilic attack. The solvents used in cationic polymerization should be inert with electrophiles and stable toward acids. Halogenated solvents like methylene chloride, methyl chloride, ethylene chloride, carbon tetrachloride, and nitro compounds like nitromethane or nitrobenzene are generally preferred solvents for cationic polymerizations.
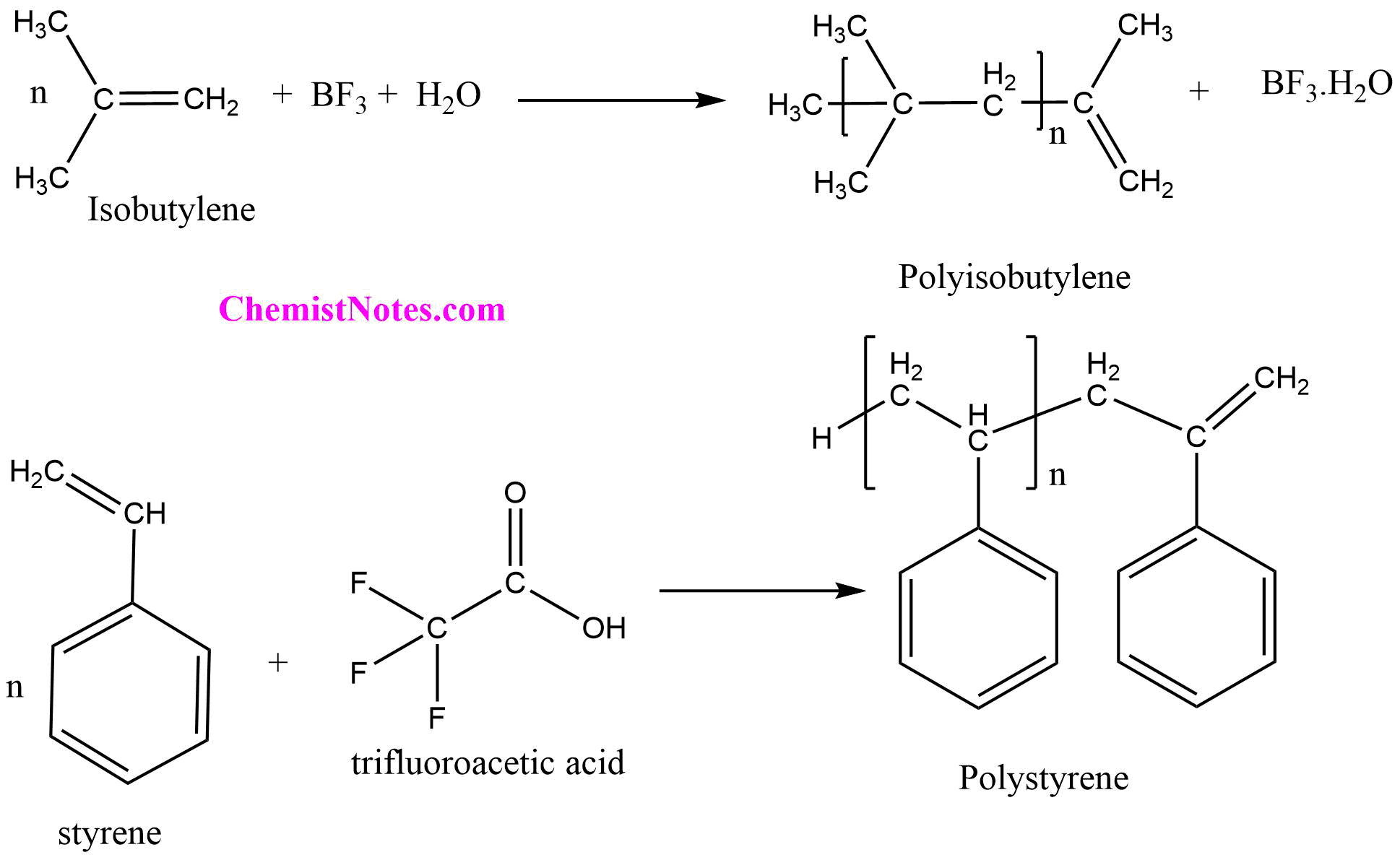
Aprotic acids (Lewis acids and Friedel-Craft halides), protic acids (Bronsted acids), and stable carbonium ion salts are typical catalysts of cationic polymerization. All these molecules are strong electrophiles. Many of these catalysts require co-catalysts (generally Lewis bases or proton donors) to initiate polymerization reactions. Polymerization of isobutylene initiated by AlCl3 and BF3 and polymerization of styrene in the presence of trifluoroacetic acid are an example of cationic polymerization.
Mechanism of cationic polymerization
The most satisfactory and widely accepted theory of cationic polymerization involves the carbonium ion as a chain initiator. The cationic polymerization occurs in three steps i.e.; initiation, propagation, and termination. To understand the mechanism of cationic polymerization let us consider the polymerization of isobutylene with boron trifluoride as catalyst.
Step I: Chain initiation
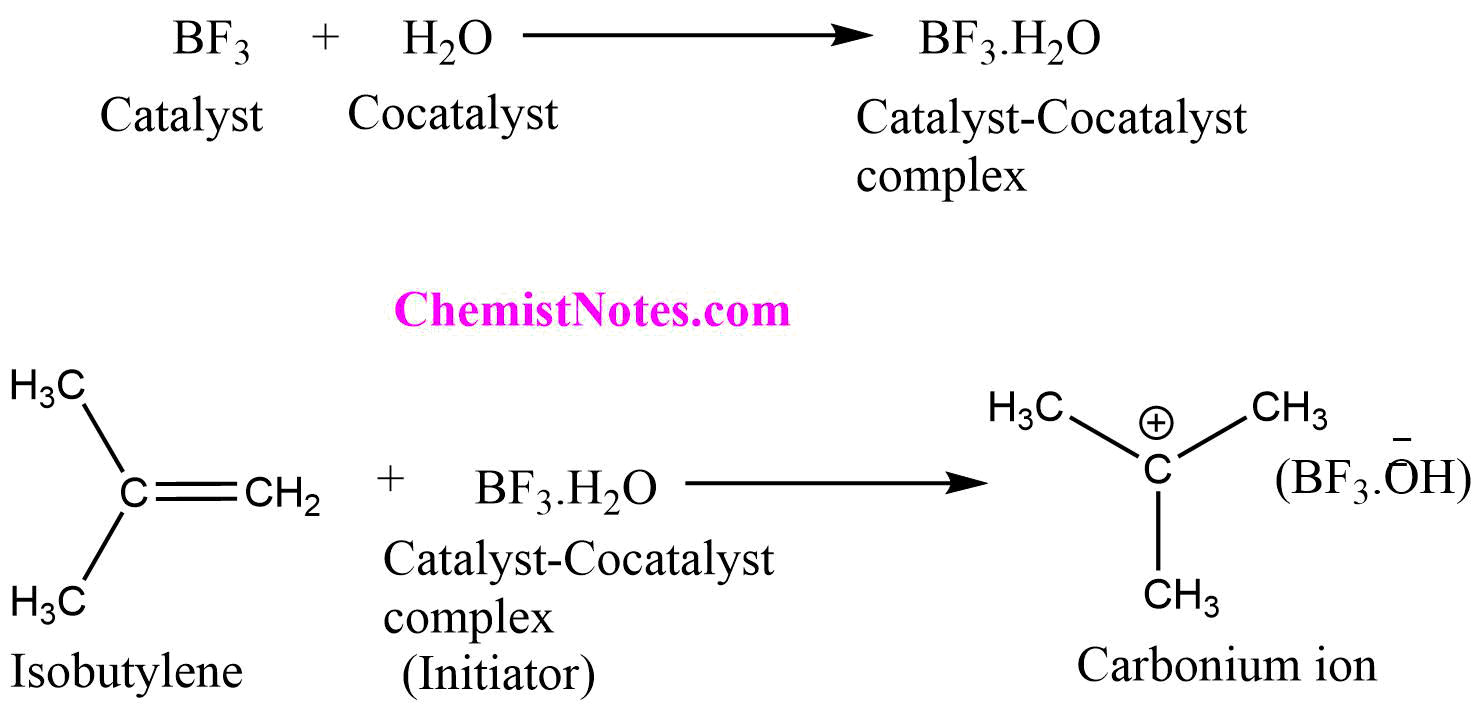
Initially the catalyst, BF3 forms a complex with co-catalyst, H2O (water). The catalyst-cocatalyst complex acts as a proton donor to an isobutylene molecule to produce carbonium ion (CH3)3C+ and initiates the chain reaction.
Step II: Propagation
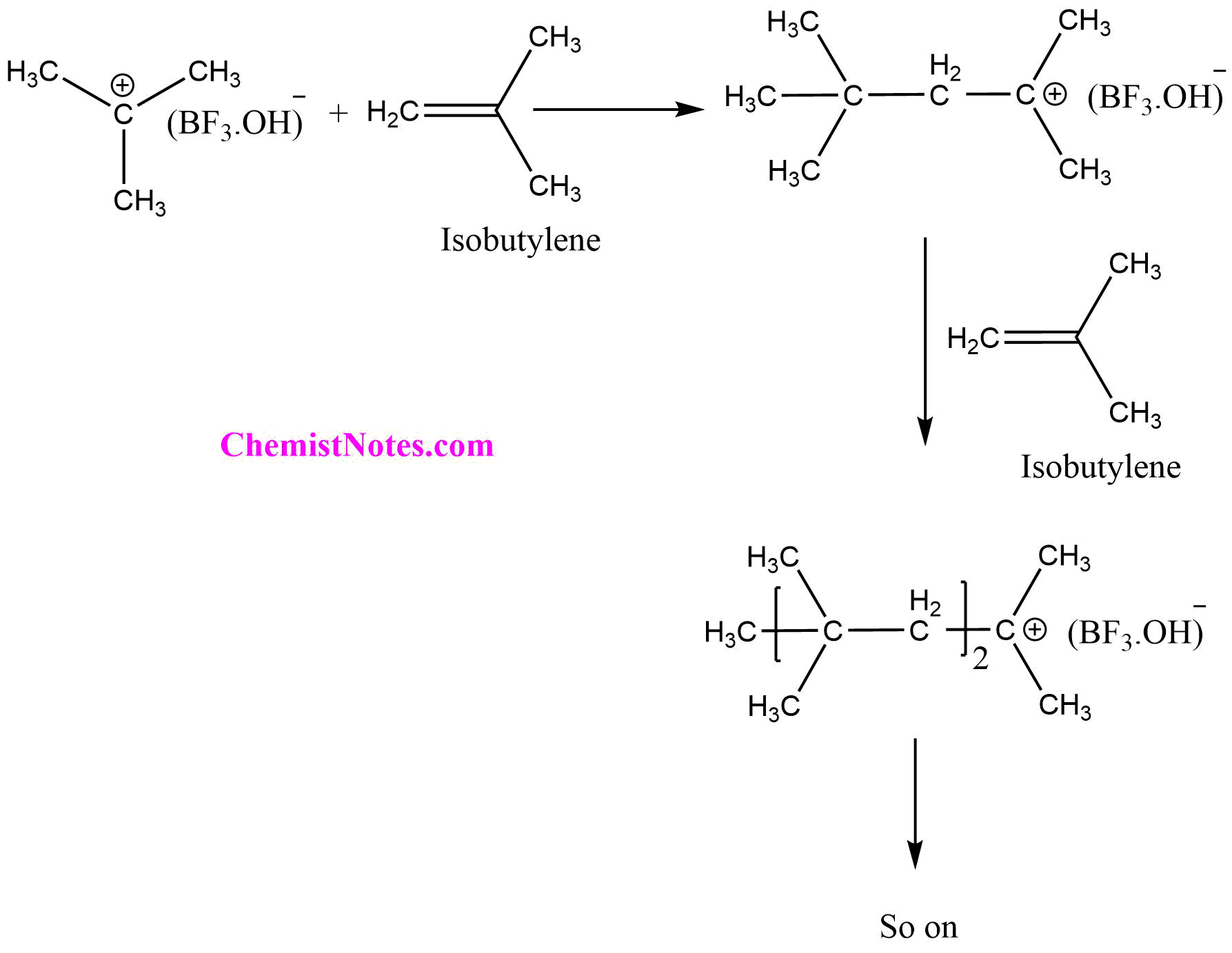
In the propagation step, the formed carbonium ion reacts with a monomer (another isobutylene molecule) regenerating the carbonium ion at the end of the reaction. The chain propagation step continues until no monomer is left or termination starts, expanding the chain of the polymer. Since, the cationic polymerization reaction is generally carried out in an aprotic solvent, having a low dielectric constant, the growing cation and initiator ion form an ion pair until the reaction is terminated.
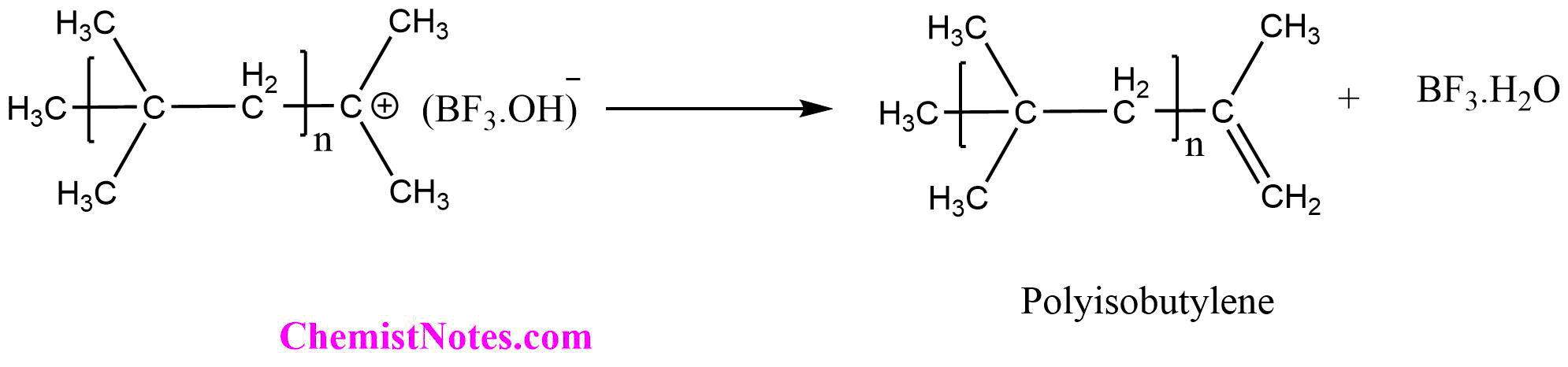
Step III: Termination
In the termination step, the rearrangement of the ion pair takes place to form a polymer with unsaturation at terminal carbon. In this step, the reaction initiator complex is regenerated.
However, an alternative mechanism of termination is observed in the polymerization of styrene with trifluoroacetic acid, in which the polymerization reaction is terminated by the formation of a covalent bond between the polymer and catalyst-cocatalyst complex.
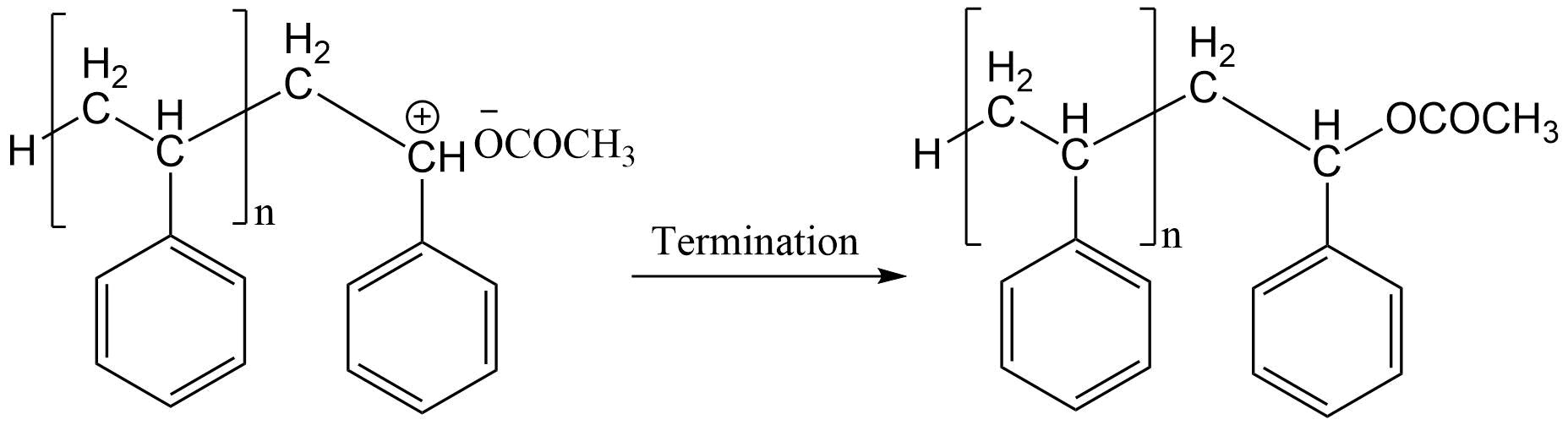
The acid strength of the catalyst-cocatalyst complex determines the efficiency of the catalyst. Also, the efficiency of the catalyst as a terminator should be related to the base strength of the complex anion. Thus, the molecule which acts as a more active catalyst is less active as a terminator.
Kinetics of cationic polymerization reaction
Although many cationic polymerization reaction proceeds so rapidly that it is difficult to establish a steady state, the following kinetic scheme has been proposed. Let us represent catalyst by A and co-catalyst by RH. The initiation, propagation, and termination steps can be represented as follows:
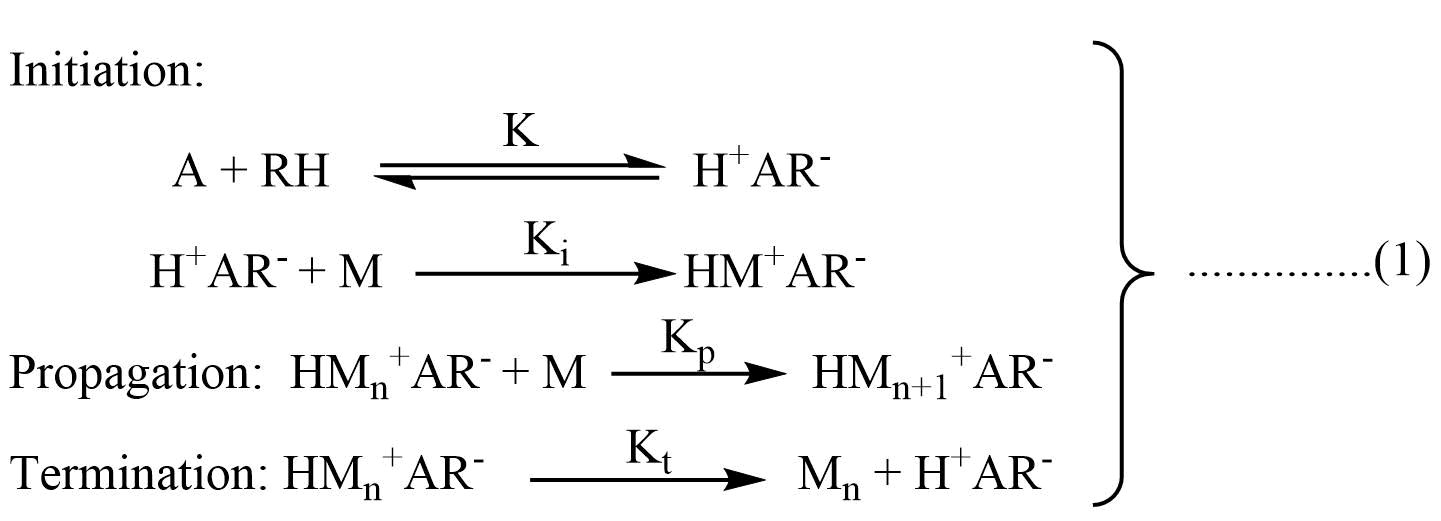
The rate of initiation is given as:
Vi = KKi[A][RH][M] …………………………(2)
Where [A] is the concentration of the catalyst, [RH] concentration of the co-catalyst, and [M] is the concentration of the monomer. If the formation of H+AR- is the rate-determining step, then Vi may be independent of [M].
Similarly, the rate of propagation and termination is given as:
Rate of propagation (Vp) = Kp[HM+nAR–] [M] …………………………………(3)
Rate of termination (Vt) = Kt[HM+nAR–] ………………………….(4)
At a steady state, the rate of initiation is equal to the rate of termination then,
KKi[A][RH][M] = Kt[HMn+AR–]
[HMn+AR–] = KKi[A][RH][M]/Kt …………………(5)
Combining equation (3) and (5), we get
Vp = KpKKi[A][RH][M]2/Kt ……………..(6)
The degree (average number) of polymerization is obtained by dividing the rate of propagation by the rate of termination.
Degree of polymerization (DP) = Vp/Vt = Kp[M]/Kt …………..(7)
Chain transfer reactions like chain transfer to monomer, spontaneous termination, and chain transfer to solvent are also involved in cationic polymerization.
In addition to combination with the counter ion, if chain transfer reactions are also present, the concentration of the propagating species remains unchanged and the polymerization rate is given as:
DP = Vp/(Vt + Vts + Vtr.M + Vtr.S) …………..(8)
The rate of spontaneous termination (Vts), transfer reaction to monomer (Vtr.M), and transfer reaction to solvent (Vtr.S) are given as:
Vts = kts[HMn+(AR)–] ……………….(9)
Vtr.M = Ktr.M[HMn+(AR)–][M] ………..(10)
Vtr.S = Ktr.S[HMn+(AR)–][S] ………….(11)
Combining equation (8) with equations (3), (4), (9), and (11), we get
DP = Kp[M]/(Kt + Kts + Ktr.M[M] + Ktr.S[S]) ……………(12)
When chain transfer to solvent terminates the reaction, the polymerization rate is decreased and is given as:
Vp = KKiKp[A][RH][M]2/ (Kt + Ktr.S[S]) …………….(13)
In the absence of any chain transfer, the kinetic chain length m is equal to the degree of polymerization and is expressed as:
V = DP = Vp/Vt = Kp[M]/Kt …………….(14)
If the chain transfer is predominant then,
V = DP = Vp/Vtr.M = Kp[HMn+(AR)–][M]/( K[HMn+(AR)–][M])
= Kp/Ktr.M ……………………(15)
Since Vp is proportional to KiKp/Kt, and if termination predominates, the degree of polymerization (DP) is proportional to Kp/Kt, the overall rate of polymerization increases with decreasing temperature if energy of termination (Et) > energy of initiation (Ei) + energy of propagation (Ep), and the molecular weight increases as the temperature decreases if energy of termination (Et) > energy of propagation (Ep).
Since, the propagation step involves the approach of an ion to a neutral molecule in a medium of low dielectric constant, no activation energy is necessary.
The termination step, on the other hand, requires the rearrangement of an ion pair and thus involves an appreciable activation energy.