Table of Contents
ToggleHomogenous and Heterogenous catalysts are mainly two types of catalysts used in organic, and inorganic chemistry.
- Homogenous catalyst
- Heterogenous catalyst
Homogenous and Heterogenous catalysts can be defined as, Heterogenous catalysts are those in which catalysts are in a different phase from that of the reactant whereas, in homogenous catalysts, the catalyst is in the same phase as a reactant.
Heterogenous catalysts
Heterogeneous catalysts are those catalysts that contain multiple parts. A heterogeneous catalyst works with reactants in different phases. Usually, heterogeneous catalysts are in a solid phase. Their reactants are in liquid or gas phases. Specific parts of a catalyst engage in different parts of a reaction. Heterogeneous catalysts are limited in their reaction capacity by the amount of surface area they have.
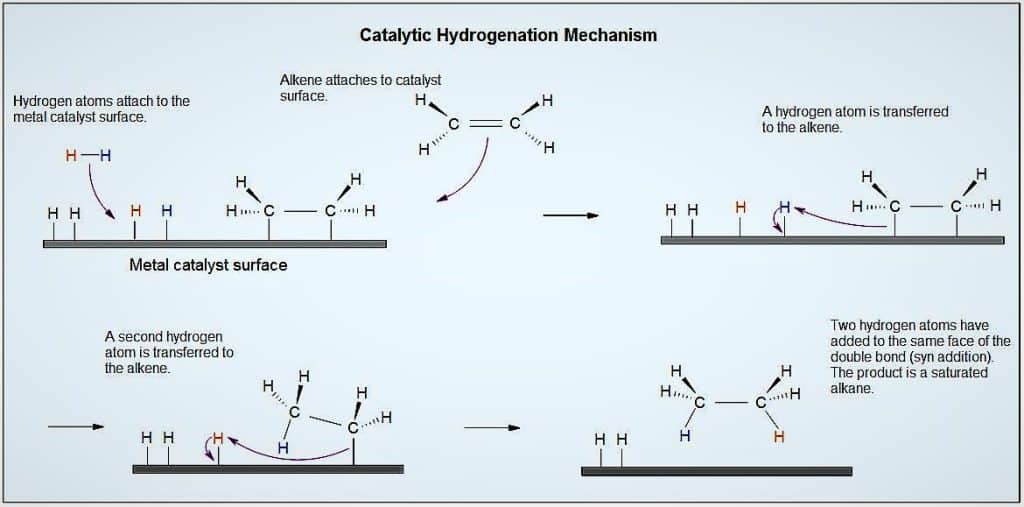
The reaction of hydrogen gas with the surface of a metal, such as Ni, Pd, or Pt, is an illustration of heterogeneous catalysis. The hydrogen-hydrogen bonds break, as seen in the picture below, causing individual hydrogen atoms to adsorb on the metal’s surface. Two hydrogen atoms can collide and form a hydrogen gas molecule, which can then leave the surface through a process known as desorption since the adsorbed atoms can move about on the surface.
In comparison to a hydrogen molecule, adsorbed H atoms on a metal surface are far more reactive. The dissociation energy of the relatively strong H-H bond, which has a dissociation energy of 432 kJ/mol, has already been broken, lowering the energy barrier for the majority of H2 reactions on the catalyst surface.
For instance, the Haber process uses the surface of iron, a solid, to catalyze the formation of ammonia (NH3).
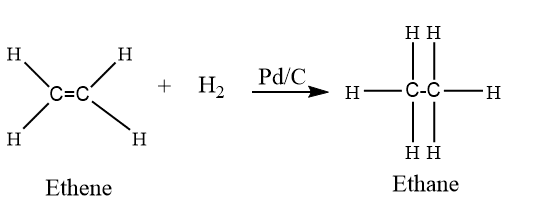
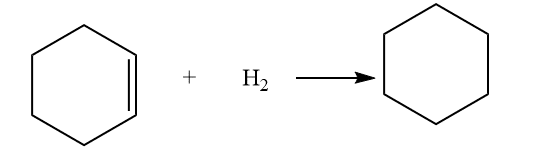
Some examples of Heterogenous catalyst
The catalysts platinum, palladium, and nickel are frequently used in the hydrogenation of alkenes. The reaction occurs on the metal catalyst, which serves as a surface. As a result of the reactants being close to one another and being able to interact more easily, the rate is increased.
1. Hydrogenation of alkene
The process of hydrogenation is an example of an alkene addition reaction. In a hydrogenation reaction, an alkene’s double bond is crossed by two hydrogen atoms to produce a saturated alkane. Due to the formation of a more stable (lower energy) product, hydrogenation of a double bond is a thermodynamically advantageous process.
In other words, it is exothermic because the energy of the product is lower than the energy of the reactant (heat is released). The heat produced, also known as the heat of hydrogenation, is a measure of how stable a molecule is.
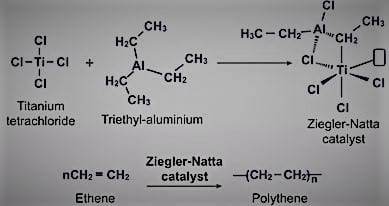
2. Zeigler Natta catalysts
It is easy to distinguish between the two main groups of Ziegler-Natta catalysts that are used based on their solubility. The catalysts are;
- Heterogeneous catalysts on support. These are catalysts made from compounds based on titanium. They are utilized in conjunction with cocatalysts and organoaluminum compounds during the polymerization operations.
2. Catalysts that are uniform. These have Hf, Ti, or Zr compounds as their foundation. Although they also have nitrogen-based and multidentate oxygen-based ligands, metallocenes are typically present in them.
The Ziegler-Natta catalyst is used in coordination polymerization reactions, which typically include complexes generated between a transition metal and a monomer’s electrons. Where the transition metal ions are bonded to the expanding chain’s terminus, polymerization typically occurs via the addition of monomers. Long polymer chains arise as a result of the simultaneous coordination of the arriving monomers at open orbital locations.
At the active center, the TiC bond also receives the C=C insertion. In the final termination stage, “dead” polymers (the desired result) are formed, which concludes the chain-growth polymerization process. These processes are also similar to anionic polymerization, which produces linear and stereo-regular polymers.
Homogenous catalysts
Homogenous catalysts are those in which catalysts are in the same phase as reactants.
A homogenous catalyst is one that is in the same phase as the reaction phase throughout the whole reaction. An illustration of this reaction is the creation of sulfur trioxide when sulfur dioxide and oxygen react with nitric oxide as a catalyst.
How does a Homogenous catalyst work?
Simply said, the catalyst is reformed during the reactions, which take place through an intermediate species. Because homogeneous catalysts can “mix-in” with the reactants, the amount of contact between the molecules of the reactants and the catalysts is very high.
Some examples of Homogenous catalysts:
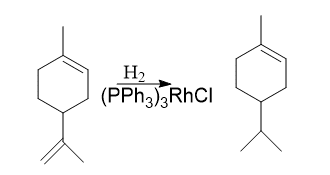
1. Hydrogenation of alkene by Wilkinsons’s catalyst
Hydrogenation of alkene by Wilkinsons’s catalyst is an example of a Homogenous catalyst involving oxidative addition and reductive elimination reaction. Wilkinson’s catalyst is RhCl(PPh3)3 – Chlorotris(triphenylphosphine)rhodium(I). It plays a vital role in homogenous hydrogenation. It is a 16-electron diamagnetic square planar complex. Rhodium in Wilkinson’s catalyst is in the +1 oxidation state and exhibits dsp2 hybridization.
Stereospecific syn hydrometallation of the multiple bonds is followed by stereospecific reductive elimination in hydrogenations catalyzed by Wilkinson’s catalyst. Therefore, olefin or alkyne hydrogenation produces syn addition products.
Currently, heterogeneous catalysis outperforms homogeneous catalysis in terms of ease and simplicity for common hydrogenation reactions. Both kinds of catalytic systems result in the suprafacial addition of hydrogen. However, homogeneous catalysts appear to be more sensitive to steric hindrance and are less likely to produce rearrangement, dissociation, and hydrogenation of other bonds, thus they can achieve higher selectivity.
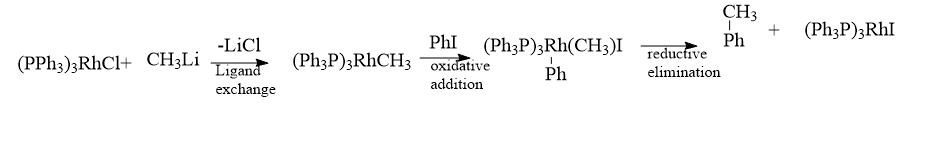
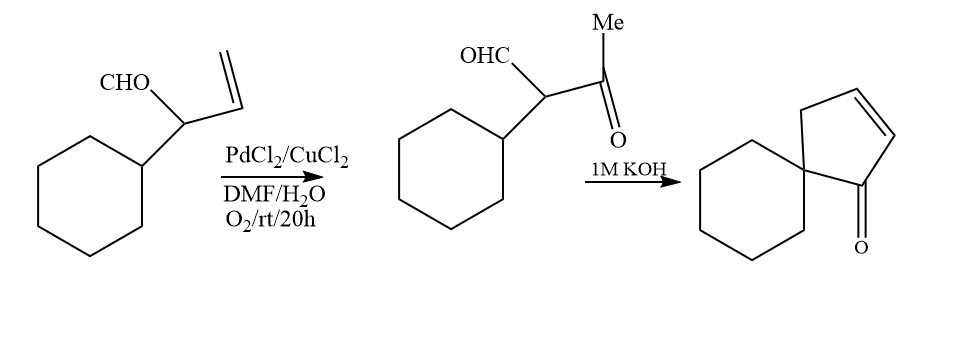
2. Wacker’s reaction
In the Wacker process, divalent palladium converts ethene to ethanal in the presence of water. The Wacker-Hoechst method has received much study, and it is used in every textbook as an illustration of a homogeneous catalyst system for nucleophilic addition alkenes. A nice example of homogeneous catalysis is the Wacker process. The palladium combination with ethylene is similar to the heterogeneous catalyst Zeise’s salt, K[PtCl3(C2H4)].
Wacker oxidation is a broad term for the reaction in which a co-oxidant, water, and catalytic palladium(II) convert a terminal or 1,2-disubstituted alkene to a ketone. Aldehydes, allylic/vinylic ethers, and allylic/vinylic amines are produced through reaction variants. The Wacker oxidation has been used extensively in organic synthesis due to the simplicity with which terminal alkenes may be produced and the adaptability of the methyl ketone group placed by the reaction.
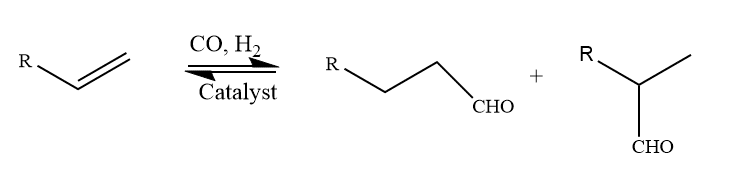
3. Hydroformylation reaction
This reaction is also an example of Homogenous catalysts. The homogeneously catalyzed industrial process of hydroformylation, sometimes referred to as oxo synthesis or the oxo process, is crucial for the generation of aldehydes from alkenes. A formyl group (CHO) and a hydrogen atom are added to a carbon-carbon double bond in this chemical process.
Advantages of Homogenous over Heterogenous catalysts
Homogeneous catalysts are advantageous to heterogeneous catalysts because they can carry out the reaction at milder conditions, have higher activity and selectivity, are simpler to spectroscopically monitor, and have controlled and tunable reaction sites. Because the catalyst and reactant molecules are in the same phase, there is a high degree of interaction during homogeneous catalysis (as opposed to heterogeneous catalysis).
Disadvantages of Homogenous and Heterogenous catalysts
The homogeneous catalyst’s frequent irrecoverability once the reaction is completed is a drawback. Thus, it may be argued that homogeneous catalysis is neither as effective nor financially feasible for industrial-scale manufacturing operations. The availability of surface area on the catalyst is a restriction of heterogeneous catalysis.
The reaction cycle cannot continue until some of the product molecules leave the surface, making room once more for a fresh batch of reactant molecules to fit on the catalyst’s surface when it is entirely saturated with reactant molecules (i.e., no more reactants can fit on the surface). Because of this, the adsorption stage is frequently the rate-limiting step in a heterogeneously catalyzed process.
MCQs/FAQs
What is a homogenous catalyst?
The process of reaction in which reactant and catalyst are in the same phase.
What is a heterogeneous catalyst?
The process of reaction in which catalysts are in a different phase than the reactant.
Why is a homogenous catalyst is more preferable over a heterogeneous catalyst?
Homogeneous catalysts are advantageous to heterogeneous catalysts because they can carry out the reaction at milder conditions, have higher activity and selectivity, are simpler to spectroscopically monitor, and have controlled and tunable reaction sites.
What are the examples of homogenous catalysts?
Examples of homogenous catalysts are wacker oxidation, Wilkinson’s catalytic reaction, and hydroformylation reaction.
What are the examples of heterogenous catalysts?
Examples of heterogeneous catalysts are the Hydrogenation of alkene, Ziegler Natta reaction, etc.
Is Wilkinson’s catalytic reaction is Homogenous catalyst?
Yes, it is a homogeneous catalyst.
What is the homogenous catalyst used for?
For chemical reactions on biopolymers, homogeneous catalysts such as transition metal complexes have been used.
Where are heterogenous catalysts used?
Heterogeneous catalysis is vital to the chemical and energy sectors. For instance, the Haber-Bosch process, which produced 144 million tons of ammonia in 2016, uses metal-based catalysts to produce the fertilizer ingredient ammonia.






