Table of Contents
ToggleEvaporation is a spontaneous natural process of a liquid turning into vapor on its surface. Because of the environmental conditions, it always occurs from the liquid’s surface where the kinetic energy of the surface molecules is higher than that of the bulk molecules. The liquid’s surface cools as a result of evaporation because the molecules that evaporate absorb the kinetic energy of the molecules that remain. A temperature increase causes an increase in the rate of evaporation.
What is evaporation?
Evaporation is the process by which a liquid transitions from the liquid phase to the gas phase. For example, when humidity influences the rate of evaporation of water, a high concentration of the material evaporating in the surrounding gas greatly slows down evaporation.
The manner in which the liquid’s molecules clash determines how much energy they exchange. In order to escape and become a gas, a molecule close to the surface must absorb sufficient energy to exceed the vapor pressure. Evaporative cooling is the process via which a liquid’s temperature is lowered by the energy extracted from the evaporated state.
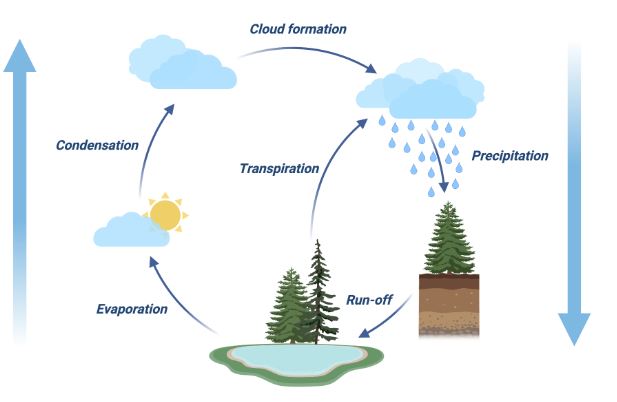
Evaporation as a part of water cycle
A crucial component of the water cycle is evaporation. The sun, or solar energy, is what causes water in lakes, seas, soil moisture, and other water sources to evaporate. In hydrology, transpiration—which is evaporation through plant stomata—and evaporation are referred to together as evapotranspiration. When the liquid’s surface is exposed, molecules can escape and turn into water vapor, which can float upward and create clouds. This process is known as evaporation of water. If there is enough energy, the liquid will evaporate.
Evaporative equilibrium
When evaporation occurs in a confined space, the molecules that are departing build up as vapor above the liquid. As the density and pressure of the vapor rise, more molecules return to the liquid, with the frequency of these returns increasing.
The vapor is considered to be “saturated” when the escape and return processes have reached equilibrium and there is no longer any potential for changes in the liquid’s temperature or vapor pressure and density. The Clausius–Clapeyron connection indicates that the equilibrium state of a system made up of the liquid and vapor of a pure material is directly correlated with the substance’s vapor pressure.
Examples of Evaporation
The examples of evaporation are explain below:
- Drying clothing in the sun: Everyone dries their clothes in the sun. This process, called evaporation, causes the moisture in the garments to evaporate and dry.
- Drying out of water bodies: We have seen that throughout the summer, evaporation causes the water in ponds and lakes to either evaporate or become less.
- Water cycle: We covered how evaporation converts water into water vapor in the previous two sections, and here is an excellent illustration of evaporation. This water vapor ascends higher into the sky, where it condenses to create clouds, which subsequently precipitate. Evaporation is therefore important for controlling the water cycle.
- Salt formation: Evaporation, the process by which water evaporates to leave behind salt, occurs naturally or artificially.
- Mopped floor drying in the summertime coolness of the desert.
Process of Evaporation
The process by which water changes from a liquid to a gaseous or vapour state is known as evaporation. The melting of an ice cube is an illustration of evaporation. The evaporation of acetone, which is used to remove nail polish, is a common example of evaporation.
Factor affecting evaporation
The factor affecting evaporation are:
- Temperature
The temperature to which it is exposed directly affects the rate of evaporation. It is exposed to more liquid at higher temperatures. Instead of the liquid, vapor is created as the kinetic energy rises. As a result, the rate of evaporation rises. It’s likely that you’ve observed that summer garments dry considerably more quickly than winter items because of the warmer temperatures.
2. Surface area
The area that the liquid is exposed to directly affects how quickly it evaporates. Let’s examine a few real-world instances. Typically, the fabric is spread out across the cloth line, increasing its surface area exposure and causing the water to evaporate. Then, for easy cooling, each of us puts our daily cup of tea into a saucer. It’s only that a portion of the particles evaporate and cause the tea to cool down.
3. Humidity
The amount of water vapor in the air has a significant impact on evaporation. The faster the material dries, the less water vapor there is. The more water vapor there is in the atmosphere, the longer it takes for the water to evaporate and dry our garments.
4. Wind speed
The rate of evaporation increases with wind velocity. The kinetic energy between the water molecules and the rate of evaporation are both increased by wind. Consider how much better a breezy day dries your clothing than a wet one.
5. Intermolecular attraction
The rate of evaporation is frequently influenced by the viscosity of substances or components. Hydrogen sulfide molecules are securely bonded, whereas water molecules are loosely bound. Therefore, compared to hydrogen sulfide, water takes less time to transition into a gaseous form.
Facts about Evaporation
- Evaporation and boiling is not same thing.
- In the absence of the evaporation process, clouds would not have formed, which would have resulted in the absence of rainfall.