Table of Contents
TogglePhotochemical reactions are quite different than thermal reactions. Simply, a photochemical reaction indicates a reaction that takes place in presence of light. The study of chemical reactions and physical changes caused by interactions between matter and visible or ultraviolet light is known as photochemistry. The production of the electronically – excited state via photon absorption is the feature that distinguishes photochemistry from other areas of chemistry.
Phtochemical reaction definition
Photochemical reactions are that reaction which take place by absorption of UV-Visible light radiation. The range of UV-Visible range in the electromagnetic spectrum is 2000 to 8000 Angstrom.
Some reactions are proceeded by absorption of heat energy from surrounding while there are other types of reactions that proceeded by absorption of energy from the light radiation, mainly of Uv-visible radiation. Those reactions that take place by absorption of heat and in absence of light are called thermal or dark reactions. Similarly, those reactions that take place by absorption of light radiation are called Photochemical reactions.
How does a photochemical reaction take place? Actually, when reactant molecules absorb Uv- Visible radiation, these molecules gain energy and get excited. Then these excited molecules undergo various photophysical processes and photochemical processes. Fluorescence and phosphorescence are photophysical processes.
Photochemistry is mainly governed by two fundamental principles. One is the Grotthus-Draper law and another is the Stork-Einstein law of photochemical equivalence.
As mentioned earlier, there are two ways that energy can be supplied to the molecules. Changing the temperature first results in a continuous increase in energy. Second, a molecule can absorb a quantum of energy from electromagnetic radiation. Thermal chemistry and photochemistry are formed as a result of these two modes of energy input.
Because a molecule’s energy levels are quantized, the amount of energy required to raise an electron from one level to a higher level in a given molecule is a fixed quantity. Only light with the exact frequency associated with this amount of energy will cause the electron to move to a higher level.
If the light of a different frequency is sent through the sample, it will pass through with no loss of intensity because the molecules will not absorb it. However, if the correct frequency of light is passed into a sample, molecules will use that energy for electron promotion, and the light that leaves the sample will be reduced in intensity or completely absent. The fate of excited molecules after the absorption of light is described by the Jablonski diagram. Similarly, to determine the efficiency of the photochemical reaction, quantum yield is calculated.
Examples of Photochemical reaction
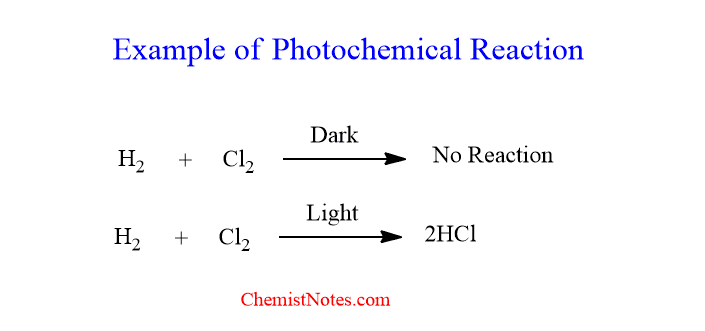
One example of a photochemical reaction is a reaction between H2 and Cl2 in presence of light. When a mixture of these reactants is kept in dark conditions, the reaction can’t take place. But when kept in presence of light, the reaction proceeds and the formation of HCl takes place with a huge sound.
Differences between Photochemical and chemical reaction
The major difference between photochemical and chemical reactions is the photochemical reaction needs light while the chemical reaction needs heat or evolve heat. Some other main differences are given below:
| Photochemical Reaction | Chemical Reaction |
| This reaction involves the absorption of light. | This reaction involves the absorption or evolution of heat. |
| The presence of light is a primary requirement for the reaction to take place. | This reaction takes place in the dark as well in the light. |
| The temperature has little effect on the rate of photochemical reaction. | The temperature has a significant effect on the rate of a chemical reaction. |
| The free energy change (ΔG) for photochemical spontaneous reactions may be +ve or –ve. | The free energy change (ΔG) for a thermochemical reaction is always negative. |
| Photochemical activation is highly selective. | Thermochemical activation is not selective in nature. |






