Table of Contents
ToggleFirst order reaction definition, examples, rate constant, integrated rate law, half-life period, graphical representation, and pseudo-first-order reaction have been discussed here.
First order reaction
first order reaction definition
First order reaction can be defined as the reaction mechanism whose rate is directly proportional to the first power of concentration of reactant. It means the first order reaction depends on the first power of concentration of reactant.
First order rate law
Let’s consider the following example of a first-order reaction.
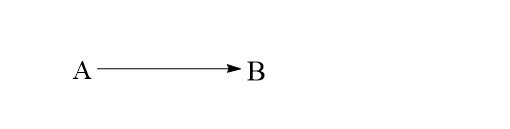
The rate law equation of this reaction is given as
Rate ∝ [Reactant]1
Rate= K [A]1=K[A]
This is rate expression for first order reaction
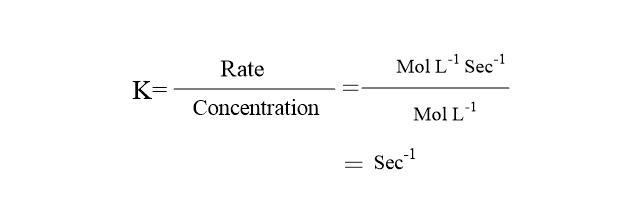
Rate constant unit of first order reaction
The unit of rate constant of first order reaction is Sec-1 or per second or per time or ( Time-1), which can be calculated as:
First order reaction example
Let’s see some examples of first-order reactions.
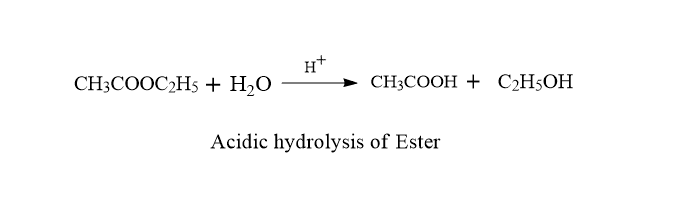
a. Acidic hydrolysis of Ester
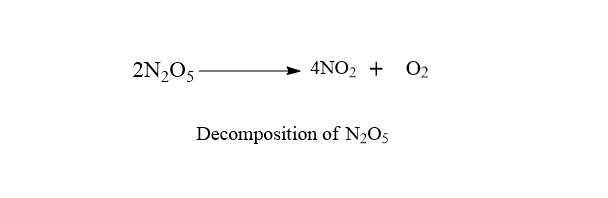
b. Decomposition of N2O5
The decomposition of dinitrogen pentoxide is a first order reaction.
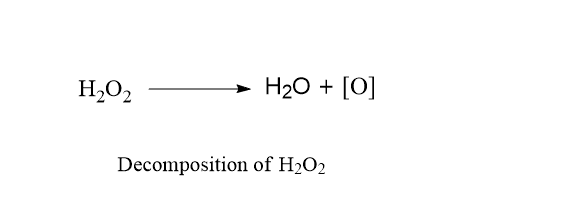
c. Decomposition of H2O2 in aqueous solution
Decomposition of H2O2 follows first order reaction
d. Radioactive decay/disintegration
All radioactive disintegration follows first-order kinetics.
Derive integrated rate law equation for first order reaction
Consider a first-order reaction,
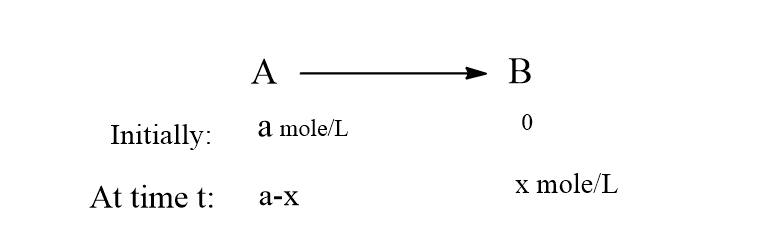
Let a mole/L be the initial concentration of reactant A. Let x be the amount of product formed at time t then (a-x) will be the amount of reactant A at time t.
From definition,
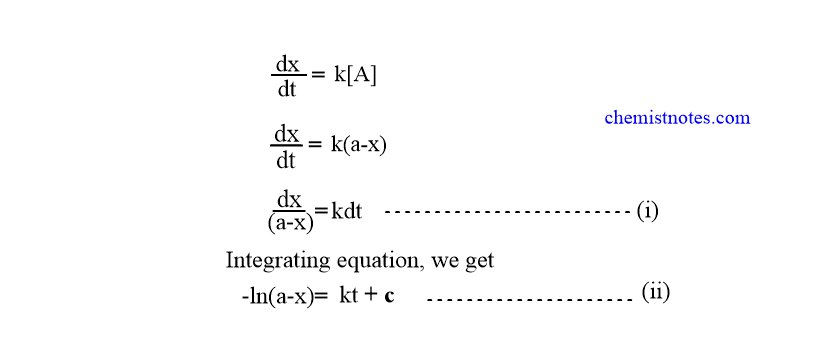
-In (a-o)= k.o + c
or, c=-In a
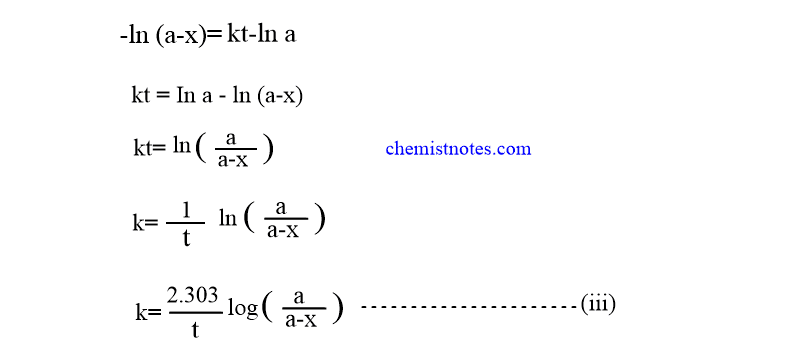
Using this value in equation (ii).
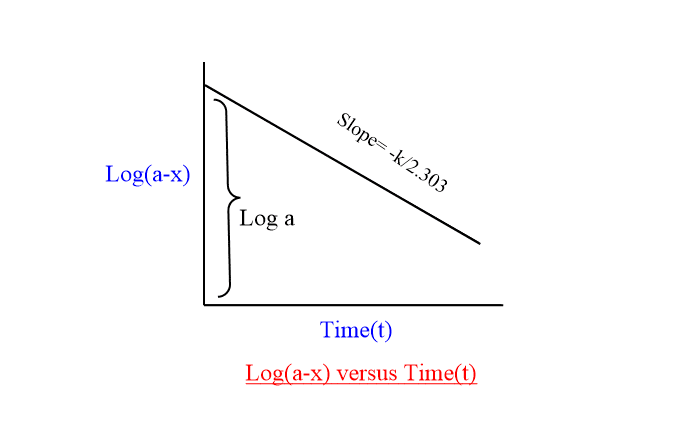
Concentration vs time graph for first order reaction
When log (a-x) is plotted against time t, a straight line will be obtained having a slope equal to (-k/2.303) and intercept log a.
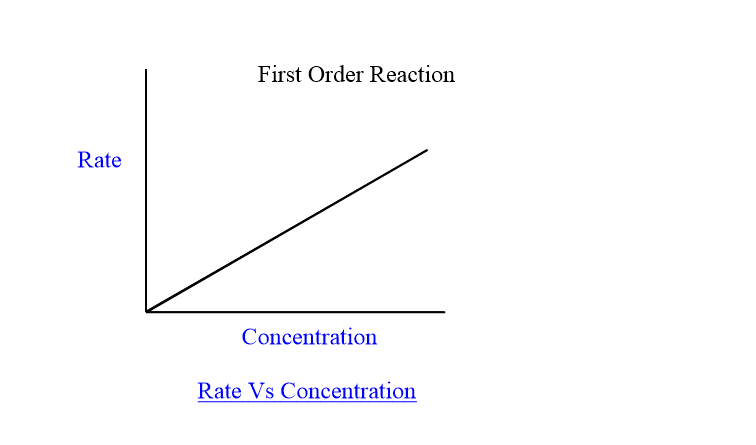
when the rate of the first reaction is plotted against the concentration of reaction, a linear line passing through origin is obtained as shown below:
Half life period for first order reaction
From the integrated rate equation,

This is the required expression of the half-life period of a first-order reaction. This shows that the half-life period of the First-order reaction is independent of the initial concentration of the reactant.
Pseudo first order reaction definition and example
Pseudo first-order reaction is defined as those reactions that seem to be of second-order or higher-order reaction but actually follow first-order kinetics. A pseudo order reaction occurs when one of the reactants is present in a large amount( excess) so that the rate is dependent on another reactant.
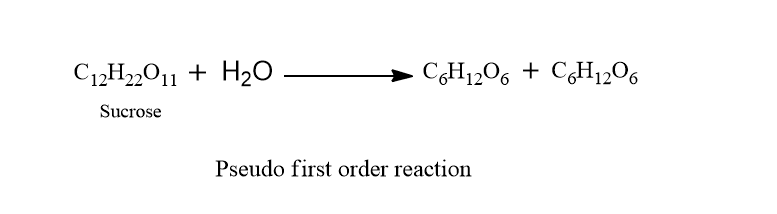
Example of pseudo first order reaction
One example of pseudo first order reaction is the hydrolysis of sucrose. In this reaction, water is present in excess amount thus rate depends only on the concentration of sucrose instead of sucrose and water.
First order reaction video
References
- P. Atkins and J. de Paula, Atkins’ Physical Chemistry, (10th Edition), Indian edition,
Oxford University Press, 2014 - K. J. Laidler, Chemical Kinetics, Harper and Row, New York, 1988.
- G. K. Vemulapalli, Physical Chemistry, Prentice-Hall of India Pvt. Ltd., New Delhi,1997.






