Table of Contents
ToggleAs per IUPAC, corrosion is defined as an irreversible interfacial interaction of materials (metallic, ceramic, or polymer) with their environment which results in the consumption of the materials. Generally, it is said to be the result of interaction between metal and environment which results in its gradual destruction. Corrosion is an undesirable phenomenon that destroys the luster and beauty of objects and shortens the life of materials. Thus, it is a destructive attack on the surface of metallic materials by chemical or electrochemical reaction with its environment leading to their degradation.
Corrosion Definition
Corrosion is defined as the undesirable deterioration of a material, usually metals or alloys by electrochemical or chemical reaction with its environment that adversely affects those properties of metals or alloys that are to be preserved. It is mainly due to the spontaneous instability of the metallic substance resulting from the charge transfer reactions at the electrified interfaces between the metallic substance and its environment.
Cause of Corrosion
- Low pH
- High temperature
- Presence of electrolyte
- Moisture
- Surface of metal itself
Types of Corrosion
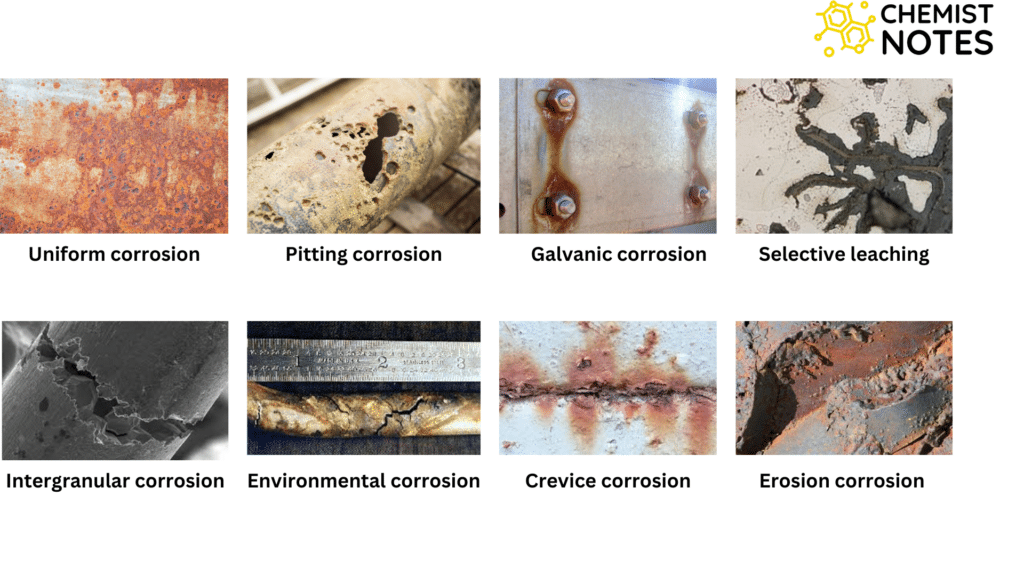
Types of corrosion based on corroded surfaces
- Uniform or general corrosion
- It is the most common form of corrosion and is characterized by a chemical or electrochemical reaction that proceeds uniformly over the entire exposed surface.
- It represents the general destruction of metallic substances like steel and is estimated that corrosion accounts for about 30% of total corrosion failure.
- However, it is relatively easy to control this corrosion by applying protective coatings, inhibitors cathodic protection, and so on.
- Pitting corrosion
- Pitting corrosion is a localized form of corrosion by which cavities or holes are produced in the materials.
- Since small pits or holes may be covered by corrosion products and often can result in sudden and unexpected failure, it is considered to be more dangerous than uniform corrosion.
- Pits can be either hemispherical or cup-shaped or any irregular shape.
- It is most aggressive in solutions containing chloride, bromide, or hypochlorite solutions.
- The presence of oxidizing agents causes the formation of pits even in the absence of oxygen.
- Galvanic corrosion
- It is the most prominent corrosion failure.
- Galvanic corrosion occurs when dissimilar metallic materials are brought into contact in the presence of an electrolyte.
- For example: when two metals with different potentials such as Cu (+0.334V) and Fe (-0.44V) are joined, a galvanic cell is formed which results in corrosion called galvanic corrosion.
- Between two different metals connected through an electrolyte, the less noble will become anode and tend to corrode in the galvanic cell.
- Selective leaching
- Selective leaching refers to the selective removal of one element or phase from an alloy by corrosion processes leaving porous materials.
- Graphitization and dezincification are examples of it.
- Dezincification is a form of corrosion in which zinc is selectively attached to zinc-containing alloys like brasses. It mainly occurs in alloys containing less than 85% copper.
- Intergranular corrosion
- The microstructure of metals and alloys is made up of grains, separated by grain boundaries.
- Intergranular corrosion is a localized attack along the grain boundaries or immediately adjacent to grain boundaries, while the bulk of the grains remains largely unaffected.
- This form of corrosion is usually associated with a chemical segregation effect or a specific phase precipitated on the grain boundaries such precipitation can produce zones of reduced corrosion resistance in the immediate vicinity.
- Environmental cracking
- Environmental cracking is a very acute form of localized corrosion.
- If metal cracks when subjected to repeated or alternate stresses in a corrosive environment, it is said to be corrosion fatigue.
- If a metal is subjected to constant tensile stress and expected simultaneously to a specific corrosion environment, cracks immediately or after a given time, the failure is called stress-corrosion cracking.
- Both stress-corrosion cracking and cracking caused by absorption of hydrogen generated by corrosion reaction causes hydrogen embrittlement.
- Crevice corrosion
- Crevice corrosion is a localized form of corrosion usually associated with a stagnant solution on the micro-environmental level.
- Such stagnant microenvironments tend to occur in crevices such as those formed under gaskets, washers, insulation materials, surface deposits, clamps, and lap joints.
- The corrosion rate of atmospheric corrosion depends on dust content, gases in the atmosphere, relative humidity, temperature, and so on.
- Erosion corrosion
- It is the acceleration in the rate of deterioration or attack on a metal because of relative movement between the corrosive fluids and metal surfaces.
- It is characterized by the appearance of growth, rounded holes, valleys, or waves.
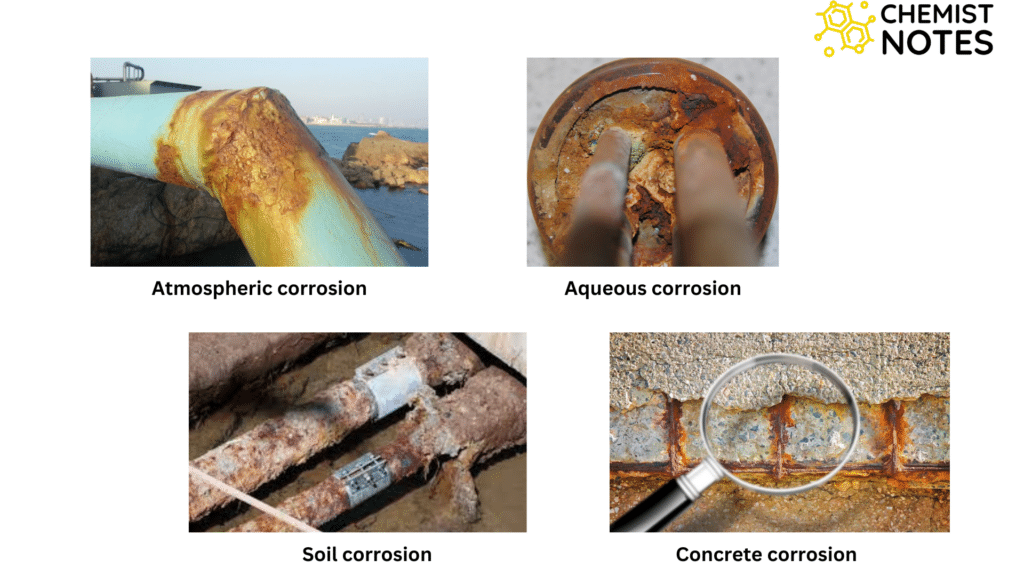
Types of corrosion based on corroded surfaces
- Atmospheric corrosion
- Atmospheric corrosion is the corrosion of materials, exposed to air, and its pollutants, rather than immersed in a liquid.
- It is an electrochemical process that depends upon the presence of electrolytes including humidity, dew, melting snow, and so on.
- Atmospheric corrosion takes place under humid conditions where the atmospheric relative humidity exceeds the equilibrium relative humidity over any saturated solution present on the material surface.
- Aqueous corrosion
- Aqueous corrosion is the corrosion of materials immersed in water.
- Due to the solvent property of water to dissolve every substance occurring in the earth’s crust and environment to some degree, water typically contains a variety of impurities that lead to corrosion.
- The corrosion rate of aqueous corrosion depends on dissolved oxygen, dissolved material ions, pH, temperature, and microbial activities.
- Soil corrosion
- Soil corrosion is the corrosion of buried steel or iron pipe for the supply of water, gas phytochemicals, etc. in the soil.
- It is the corrosion of structural materials mostly steel, iron, or stainless steel in the soils.
- Soil corrosion of different materials varies with the types of soil.
- The corrosion rate of soil corrosion depends on soil composition, moisture content, pH, resistivity, chemical species microorganisms, and so on.
- Concrete corrosion
- Concrete corrosion is the corrosion of materials such as iron, steel, etc. embedded in the concrete.
- It occurs in all concrete structures such as bridges, buildings, highways, tunnels, dams, etc.
- Concrete is a complex composition of materials that possesses high compressive strength, and is responsible for the corrosion of metallic materials.
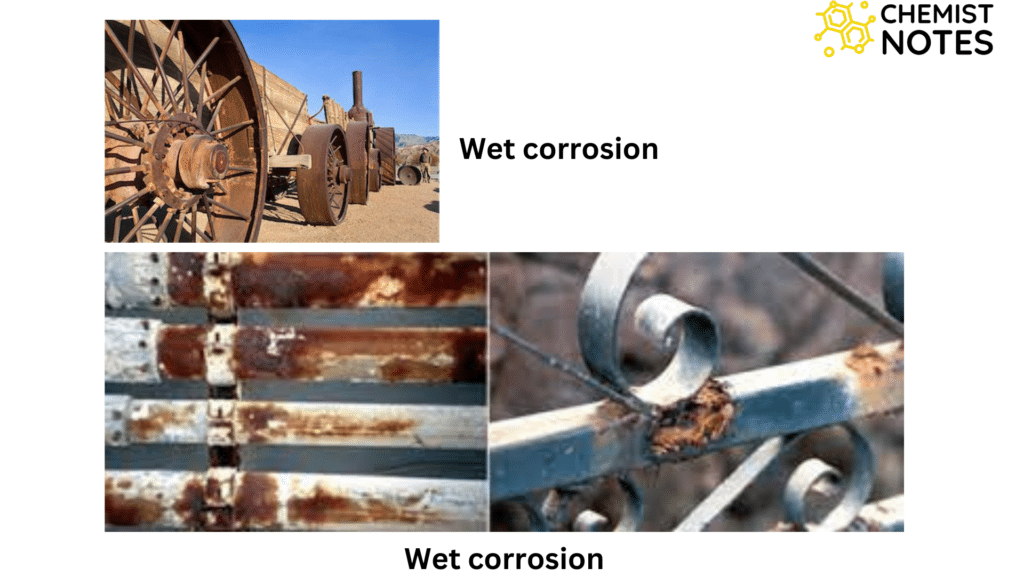
Types of corrosion based on mechanism
- Dry corrosion
- It is chemical in nature.
- Dry corrosion is caused by the interaction of metals with atmospheric gases such as oxygen, H2S, halogens, SO2, and oxides of nitrogen.
- It is mainly due to the presence of oxygen.
- When metal is in close contact with oxygen or gases like H2S, halogens, and so on, its color turnishes due to the formation of metal-oxide film, halides, sulfides, etc.
- Dry corrosion is the attack of dry gases on the metal surface which deteriorates the metal.
- It occurs at ambient temperature but increases with an increase in temperature.
- Wet corrosion
- It is an electrochemical in nature.
- It occurs in an aqueous environment.
- Rusting of iron is a kind of wet corrosion.
- In wet corrosion, metal surface in contact with moisture gets oxidized into metal ions.
- In the metal surface contact with air acts as a cathode where air is reduced into hydroxide or hydrogen ions are reduced into hydrogen gas. In such instances, electrons flow from the anode to the cathode.
Corrosion Control Method
The corrosion process is controlled by metallic coating as it protects the metallic substrate either by acting as a barrier between the environment and substrate or by corroding preferentially with reference to the substrate.
- Electro galvanizing (Zinc coating): Zinc protects the steel substrate from corrosion because Zn has a -0.76V potential as compared to iron (-0.44V). As a result, Zn is oxidized to ZnO in dry air and ZnO further reacts to form Zn(OH)2 in the presence of moisture. Thus, formed Zn(OH)2 may further react to form a protective film of ZnCO3.
- Nickel and Chromium coating: Nickel and chromium coatings are used to prevent corrosion and provide a smooth surface for steel parts when they are in contact with a corroding environment. In order to make a surface brighter and more corrosion-resistant, the nickel plate is covered by a thin coating of Cr. The Chromium coating has a large number of microspores which makes the corrosion of nickel more feasible. The Cr layer is usually deposited on top of a thin layer of Ni where Ni is in anodic relation with respect to Cr and the metal substrate.
- Lead coating: Generally, lead is used in a coating that is exposed to dilute sulfuric acid in an industrial atmosphere. It is reported that Pb coating is usually more resistant to atmospheric attack. Some of the application of lead coating involves roofing and protection of automobiles gasoline and tanks from corrosion. It should be noted that lead coatings are not protective in soil. Moreover, it should not be used in contact with food products and water since it is poisonous in nature.
Importance of Corrosion Study
Corrosion study becomes very important in terms of the following perspective:
- Economy factor: Corrosion widely impacts in economy since it destroys many materials directly associated with people’s wealth. With the help of corrosion study, these materials can be preserved, and hence the economy can be prevented from declining.
- Material designing: Corrosion study is of great importance in engineering to design materials so that corrosion can be minimized and loss of materials ultimately reduced. Designing materials with knowledge of corrosion can also increase efficiency, reduce contamination, and shut down equipment.
- Preservation of materials: The metals like iron, aluminum, copper, etc. can be preserved with knowledge of corrosion study. Corrosion engineers with the support of corrosion knowledge reduce material losses, thereby enhancing economic growth. Via corrosion study, the corrosion of piping, tanks, metal components of machines, ships, bridges, marine structures, etc. can be preserved.