Table of Contents
ToggleDiffusion controlled reaction
Diffusion controlled reactions are reactions in which the reaction rate is equal to the diffusion rate of reactants through the reaction medium such as gas or liquid. In these reactions, the rate at which reactants are transported through the medium controls the reaction rate. Therefore, these are also known as diffusion-limited reactions.
These reactions occur in a wide range of situations from biological systems to nuclear reactions at low reactant concentrations. At low concentrations, the chances of contact between reactant molecules with each other are low. In this situation, the reaction rate is determined by the pace at which these reactant molecules come closer in contact.
Diffusion-controlled reaction in solution
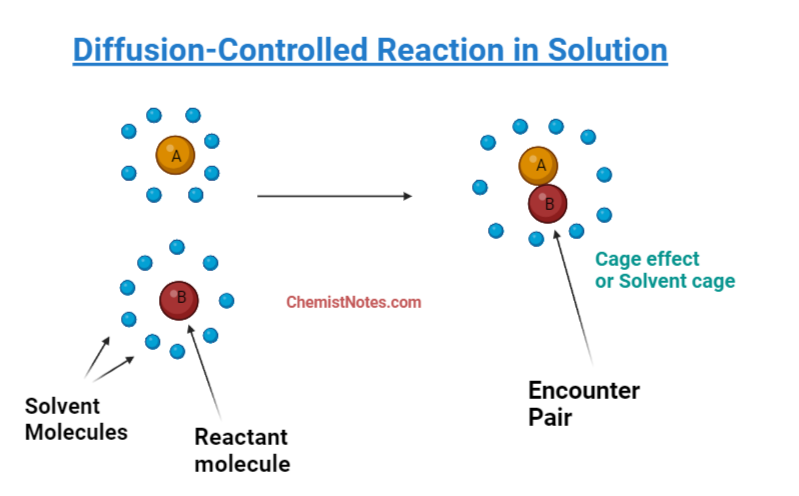
One example of a diffusion-controlled reaction is the reaction between two reactants in a dilute solution. Reactants diffuse until they come into contact with one another in the proper stoichiometry and produce the product species. In solution, the reactant molecules must diffuse through the solvents, they come into contact with each other much less frequently than in gas. Two reactant molecules that come into contact stay close to one another for significantly longer. Therefore, reactant interactions in solution take place considerably differently from those in gases.
There is one effect observed in the solution during the diffusion of reactant molecules, known as the cage effect in which the lingering of one molecule near another molecule takes place due to hindering of solvent molecules. This term can be defined as “When two different particles end up near each other in solution, they may be trapped as a result of solvent particle surrounding them, which is known as cage effect or solvent cage.”
Diffusion Controlled reaction equation
Let the rate of formation of an encounters pair AB be first order in each of the reactants A and B.
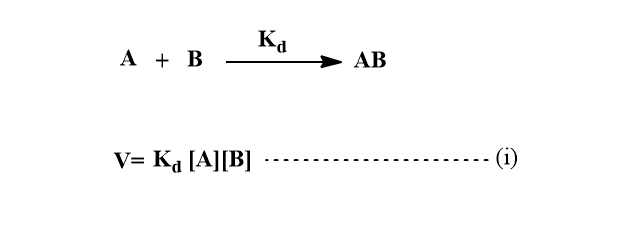
Where Kd is determined by the diffusional characteristics of A and B.
The encounters pair can break up without undergoing reaction or it can go on to form product P. If we suppose both processes are pseudo-first-order reactions with the solvent perhaps playing a role then,
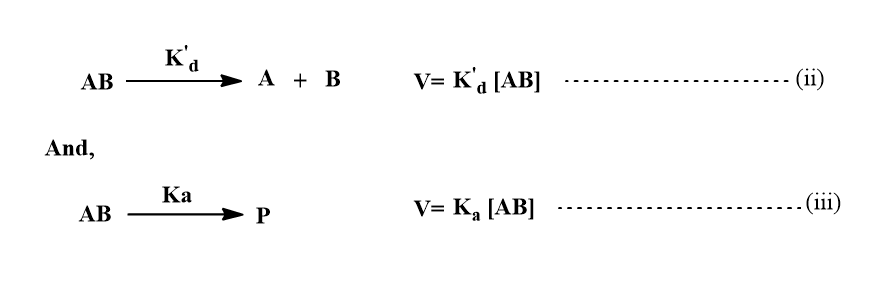
Now, the net rate of change of concentration of AB
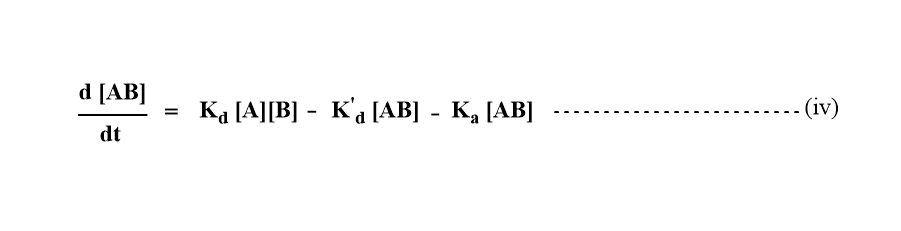
On applying the steady-state principle or approximation,
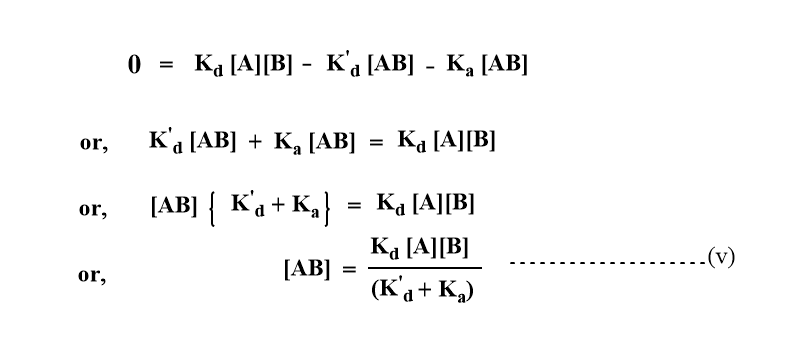
Now, the rate of formation of the product is,
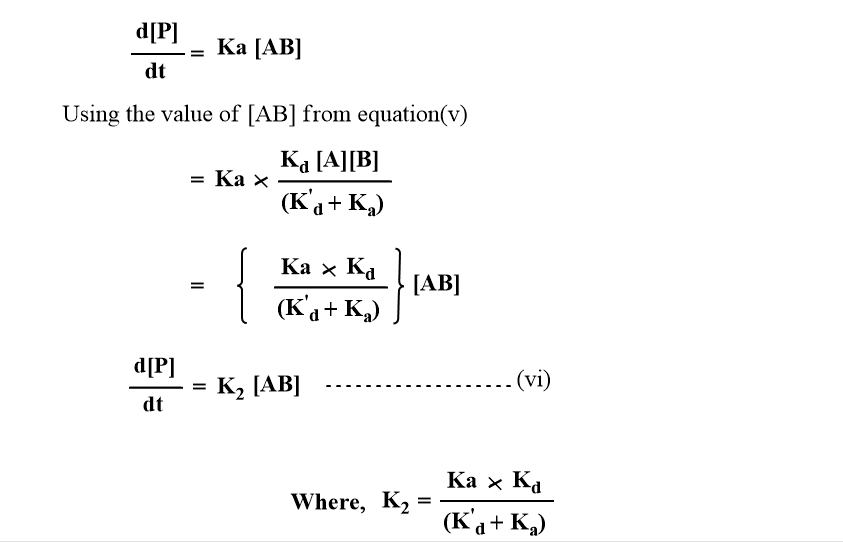
Two limits can be distinguished for the above equation(vi)
Case I: If the rate of product formation is greater than the rate of separation of unreacted encounter pair i.e Ka>> Kd’ then,
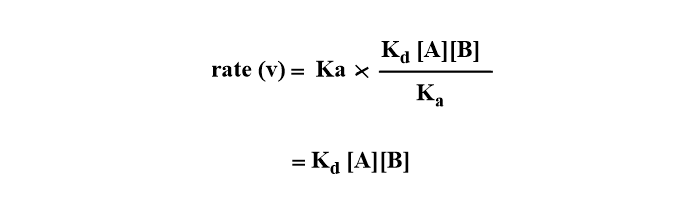
The rate of reaction is governed by the rate at which reactant molecules diffuse through the solvent i.e diffusion-controlled reaction.
Case II: If the rate of separation of unreacted encounter pair is greater than the rate of product formation i.e Kd’>> Kd then,
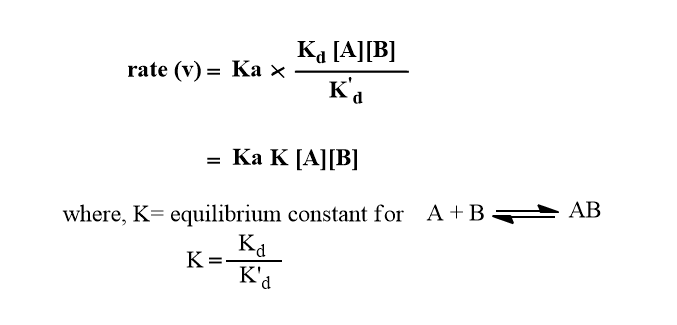
The reaction proceeds at the rate at which the energy accumulates in encounter pair from surrounding i.e. activation controlled reaction.
Somoluchowski’s equation for rate constant
Using Fick’s law, Somoluchowski derived the equation for the rate constant as,
Kd= 4πR*DNA ……………………………………(Vii)
Where,
R*=distance between two molecules
NA= Avogardo’s Number
Here, D = DA + DB = Sum of diffusion coefficient
DA= diffusion coefficient of Reactant A
DB= diffusion coefficient of Reactant B
Now, using Stoke’s-Einstein equation, we get,
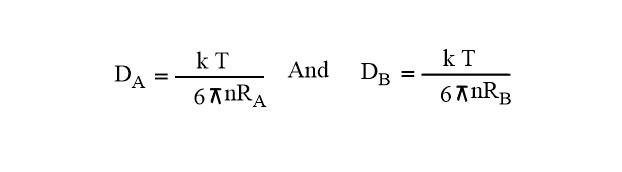
Assuming RA=RB= R*/2
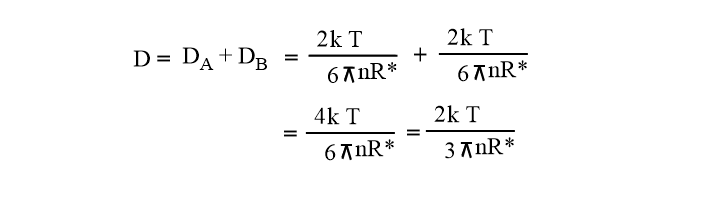
Equation (vii) becomes,
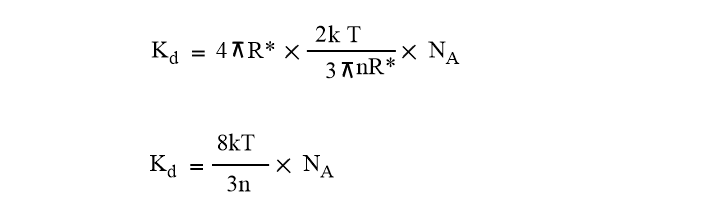
Also, we have K= R/ NA =Boltzmann’s constant
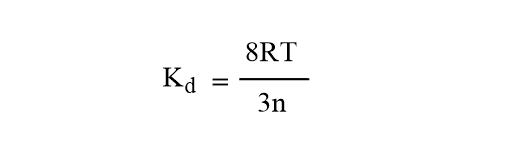
This expression shows that the rate constant is independent of the reactant molecule but depends only on the temperature and viscosity of solvents.
FAQs/MCQs:
what is diffusion controlled reaction?
Diffusion controlled reactions are reactions in which the reaction rate is equal to the diffusion rate of reactants through the reaction medium such as gas or liquid.
what is activation controlled reaction?
If the reaction proceeds at the rate at which the energy accumulates in encounter pair from surrounding, the such reaction is known as activation controlled reaction.