Table of Contents
ToggleUv visible spectroscopy refers to Ultraviolet-visible spectroscopy, which is an important tool in chemistry. It involves the promotion of the electrons from the ground state to the higher energy or excited state by absorbing ultraviolet or visible light. The range of UV-visible range extends from 100 nm to 700 nm, in which the actual Uv range is 100-400 nm and the visible range is 400-700 nm.
The absorption of Uv-visible radiation by a molecule leads to transitions among the electronic energy levels of the molecules.Therefore, it is also known as Electronic spectroscopy.
Basics of uv visible spectroscopy
The fundamental principle of UV-visible spectroscopy is the interaction of radiant energy with matter.
UV-Visible spectroscopy is based on the Beer-Lambert law, which states that when a beam of monochromatic light is passed through a solution of an absorbing substance, the rate of decrease of intensity of radiation with the thickness of the absorbing solution is proportional to the intensity of the incident radiation as well as the concentration of the solution.
i.e. A=Ecb=log(Io/I)
Where A= absorbance
Io=intensity of incident light
I= intensity of transmitted light.
E= molar absorptivity
C= concentration of sample(mole/L)
L= path lengh (cm)
From Beer-lambert’s law, it is clear that the greater the number of molecules capable of absorbing the light of a given wavelength, the greater the extent of light absorption. This is the basic principle of UV-Vis spectroscopy.
When molecules absorb UV-Visible light, it undergoes an electronic transition. Since the energy levels of molecules are quantized, the energy required to bring the excitation is a fixed quantity. Thus, the electromagnetic radiation with only a particular value of frequency will be able to cause excitation. The amount of radiation absorbed is characteristic of a substance.
Are you familiar with Woodward-Fieser rule? if not Read this rule which helps to predict the position of absorption maxima in the case of diene as well as triene compounds.
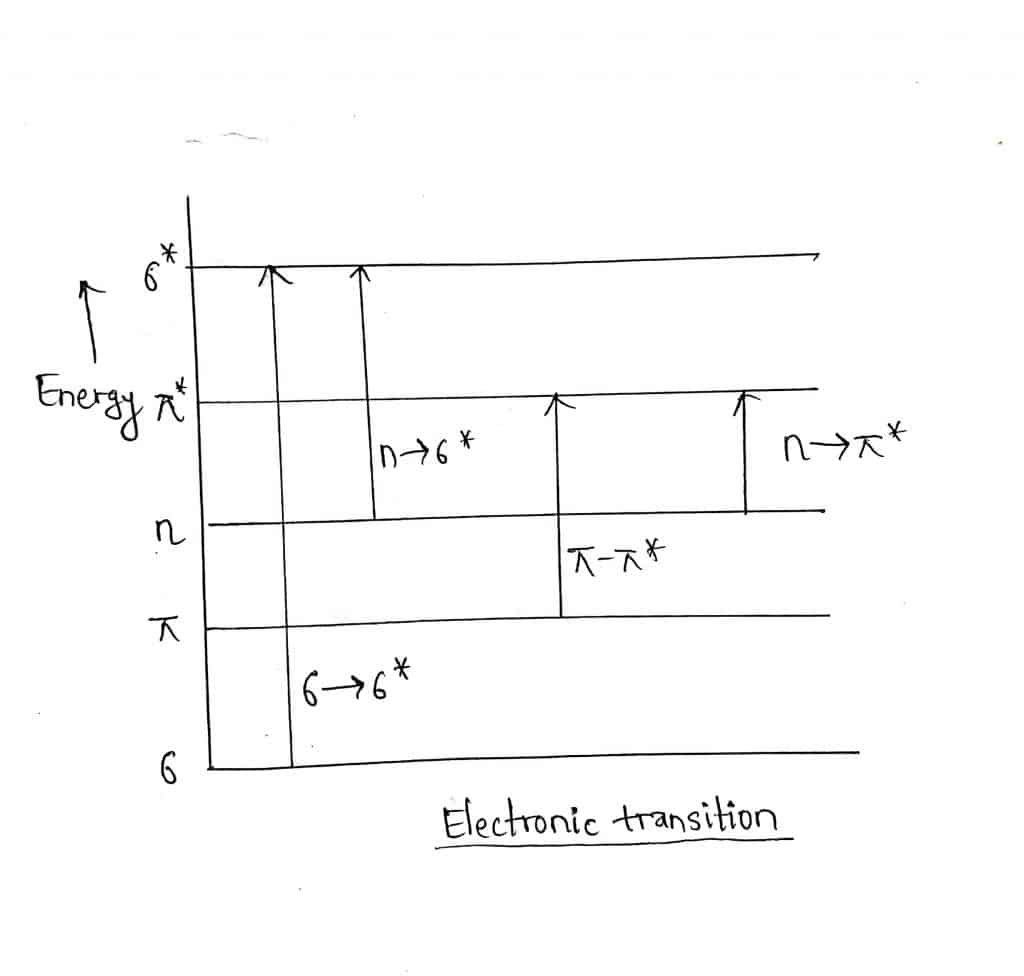
Types of electronic transition in uv visible spectroscopy
When a molecule absorbs UV-Visible light, there is the possibility of four types of electronic transitions.
- σ to σ* transition: A high amount of energy is required for this type of transition. It occurs in saturated hydrocarbons like methane, propane, etc.
- n to σ* transition: Compounds having lone pair of electrons undergo this transition. For example alcohols, ethers, aldehydes and ketones, etc.
- n to π* transition: Less amount of energy is required for this transition as shown by compounds having a double or triple bond. For example; saturated aldehydes, etc.
- π to π* transition: Such transition occurs in the unsaturated center of molecules and also in aromatics compounds. It requires slightly greater energy than n to π. For example Ethene, ethyne, other alkene and alkynes.
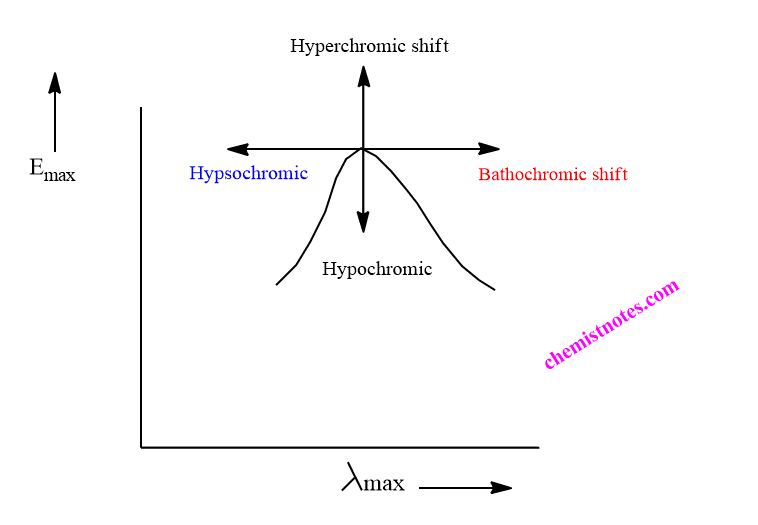
Redshift and blueshift in uv visible spectroscopy
Redshift and blueshift are also known as Bathochromic shifts and Hypsochromic shifts respectively. Not only redshift and blueshift but there are also altogether four types of shifts in UV visible spectroscopy.
- Bathochromic shifts: Bathochromic shift is an effect by the virtue of which the absorption maximum is shifted towards the longer wavelength due to the presence of an autochrome or change in a solvent. It is also called redshift. n to π transition of carbonyl compounds experiences bathochromic shifts.
- Hypsochromic shift: Hypsochromic shift is an effect by virtue of which absorption maximum is shifted towards the shorter wavelength. It may be caused due to the removal of conjugation or by changing the polarity of the solvents. This effect is also known as the blue shift.
- Hyperchromic effect: Hyperchromic shift is an effect by the virtue of which intensity of absorption maximum increases. The introduction of an autochrome in the compounds generally results in the hyperchromic effect.
- Hypochromic effect: Hypochromic effect is defined as the effect by virtue of the intensity of absorption maximum decreases. The hypochromic effect occurs due to the distortion of the geometry of the molecules with the introduction of the new group.
Application of uv visible spectroscopy
The major application of this spectroscopic technique are given below:
Functional group detection
Uv visible spectroscopy is used to detect the presence of certain functional groups with the help of the UV spectrum. Similarly, it is used to detect the presence or absence of chromophores in the component.
Detection of extent of conjugation
It is useful to detect the presence of conjugation in the compounds. The more extensive the conjugation, the longer the wavelength of the absorption.
Identification of an unknown compound
Compounds having similar structures have similar absorption spectra. The spectrum of an unknown compound is compared with the spectrum of a reference compound and if both spectrums coincide, it confirms the identification of the unknown substance.
Determination of configuration of geometrical isomers
Trans isomer exhibits λmax at a slightly longer wavelength than cis isomer. The cis-isomer suffers distortion and absorptions at a lower wavelength than trans-isomers.
Moreover, UV visible spectroscopy is used for detecting the impurities in organic compounds i.e. it can be used for the determination of the purity of a substance.






