Table of Contents
ToggleNormality is the number of gram-equivalents of solute present in one liter of the solution. It is a necessary term used in volumetric analysis and is represented by N, i.e.,
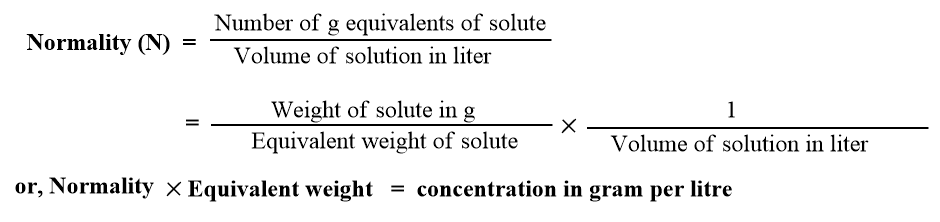
A solution having normality equal to one is called a normal solution. Such a solution contains one gram equivalent of solute per liter of the solution.
Normality Equation

In this reaction NaOH and HCl react with each other in the ratio of 40:36.5 which is equal to the ratio of their equivalent weights. Since, 1000 cc of 1N-solution of a substance contains one gram equivalent of it, hence 40g of NaOH and 36.5g of HCl would be present in their respective 1000 cc of 1N solution. On this basis,
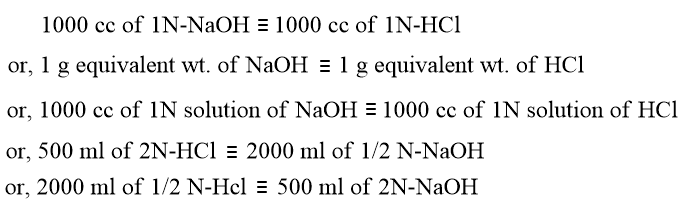
Hence, it can be concluded that
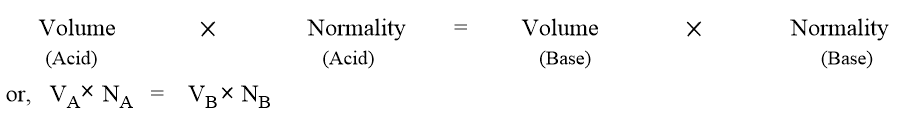
This is called normalityy equation. It is also expressed as;
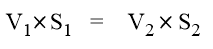
Normality Factor
It is quite difficult to correctly weigh out the required amount of a substance. As a result, we often weigh the quantity that is close to the required amount. The normality factor is defined as the ratio of actual weight of the solute taken to the theoretical weight to be taken, i.e.,
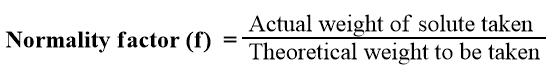
Exact Normality = Normality factor × Normality
For example, for the preparation of 250 ml N/10 solution of anhydrous sodium carbonate we have to dissolve exactly 1.3250 g of it in 250 ml. Suppose, we dissolve 1.3168 g, then
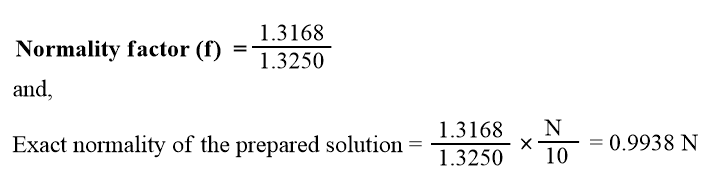
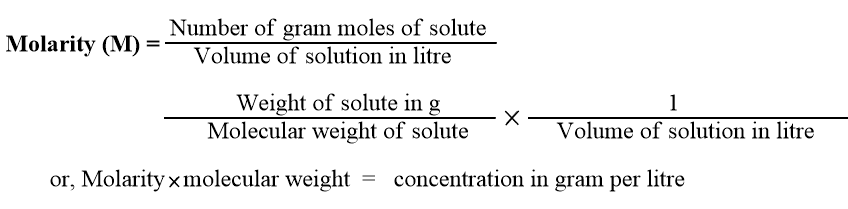
Molarity
It is defined as the number of gram-moles of solute present in one litre of the solution. It is represented by M, i.e.,
A solution having molarity one is called molar solution. Such a solution contains one gram mole of solute in one litre of the solution.
Some values of molarity are:
Decimolar:
M/10 = 0.1 M
Semimolar:
M/2 = 0.5 M
Pentimolar:
M/5 = 0.2 M
Centimolar:
M/100 = 0.01 M
Millimolar:
M/1000 = 0.001 M
Convert Normality to Molarity
The easiest formula to calculate normality using molarity is:
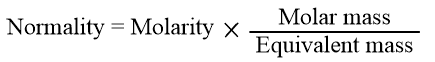
For some solutions, Normality and Molarity are equivalent or N=M. This occurs when N=1.
For acidic solutions, normality can be calculated as:
Normality = Molarity × Basicity
Here, basicity refers to the number of H+ ions that can be given by an acid molecule.
For bases, normality can be calculated as:
Normality = Molarity × Acidity
Acidity is the number of OH– ions that can be given by a base molecule.
Normality Vs Molarity
| Molarity | Normality |
| Molarity is the number of moles of a compound present in a litre of a solution. | The normality of a solution is the gram-equivalent weight of a solute in one litre of solution. |
| Unit is mol/L | Unit is eq/L or meq/L |
| Temperature changes can change the molarity of a solution by increasing the volume. | The temperature has no effect on the normality of a solution. |
| The molarity of a solution depends on the temperature, volume, addition of more solutes, and the solubility of a solute. | Normality of a solution reactive species that is present in that solution. |
| M = mol/L | N = M × n |






