Table of Contents
ToggleRedox equations play a crucial role in understanding chemical reactions. They represent the transfer of electrons between species and help us to understand chemistry.
However, balancing redox equations can often be a difficult task, especially when dealing with complex reactions involving multiple elements. Fortunately, with the advent of redox reaction calculators, this process has become significantly easier and more efficient.
Understanding Redox Reactions
Redox reactions, short for reduction-oxidation reactions, involve the transfer of electrons from one species to another. The species that loses electrons is oxidized, while the species that gains electrons is reduced. Balancing these reactions is essential for accurately representing the chemical changes occurring.
Challenges in Balancing Redox Equations
Balancing redox equations can be challenging due to several factors. Complex reaction equations, unequal numbers of atoms and charges on both sides, and multiple elements undergoing oxidation or reduction further complicate the process. This is where redox reaction calculators come to the rescue.
Introduction to Redox Reaction Calculator
A redox reaction calculator is a powerful tool designed to simplify the balancing of redox equations. It automates the process, saving time and reducing errors. By inputting the reactants and reaction conditions, the calculator can swiftly determine the balanced equation.
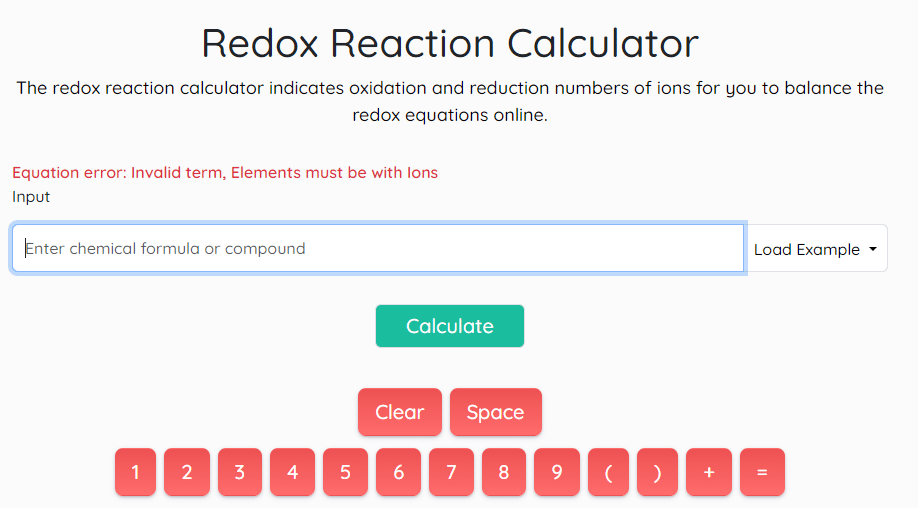
Using a redox reaction calculator offers numerous benefits. It provides instant results, eliminates tedious manual calculations, and ensures accurate balancing, even for complex reactions.
Whether you are a student, a researcher, or a chemistry enthusiast, utilizing a redox reaction calculator can significantly enhance your understanding of redox reactions.
Step-by-Step Guide: Balancing Redox Equations Using a Redox Reaction Calculator
Now let’s see a step-by-step guide on how to balance redox equations using a redox reaction calculator. By following these instructions, you’ll be able to effortlessly balance even the most intricate redox reactions.
Step 1: Identifying the Oxidized and Reduced Species
Begin by identifying the species that undergo oxidation and reduction. The oxidized species loses electrons, while the reduced species gains electrons.
Step 2: Determining the Oxidation Numbers
Assign oxidation numbers to each element in the equation to track electron transfer. The oxidation number represents the charge an atom would have if the shared electrons were completely transferred.
Step 3: Writing Half-Reactions
Split the redox equation into two half-reactions: the oxidation half-reaction and the reduction half-reaction. This separation allows you to balance each half separately.
Step 4: Balancing Atoms
Balance the atoms in each half-reaction by adding coefficients as needed. This step ensures that the number of atoms is the same on both sides of the equation.
Step 5: Balancing Charges
Balance the charges in each half-reaction by adding electrons. This adjustment guarantees that the total charge is the same on both sides of the equation.
Step 6: Balancing Electrons
Equalize the number of electrons transferred in both half-reactions by multiplying the half-reactions by appropriate coefficients.
Step 7: Combining the Half-Reactions
Combine the two half-reactions by canceling out the electrons. This step allows you to obtain the overall balanced redox equation.
Step 8: Confirming the Balanced Equation
Verify that the final equation is balanced in terms of both atoms and charges. Double-check all coefficients and charges to ensure accuracy.
Tips and Tricks for Using a Redox Reaction Calculator
While using a redox reaction calculator simplifies the balancing process, it’s essential to be aware of potential pitfalls and maximize its potential. Consider the following tips:
Understanding common pitfalls
Be mindful of common mistakes, such as overlooking oxidation state changes or failing to balance charges properly. Familiarize yourself with the calculator’s features to prevent errors.
Adjusting reaction conditions
Some redox reactions occur in acidic or basic solutions. Redox reaction calculators often allow you to specify the reaction conditions, enabling accurate balancing for different scenarios.
Utilizing advanced features
Explore the calculator’s advanced features, such as the ability to calculate reaction enthalpy, equilibrium constants, and other parameters. These additional functionalities can depend on your understanding of the redox reaction.
Example of Balancing Redox Equations Using a Redox Reaction Calculator
Let’s consider a few examples to illustrate the efficiency of redox reaction calculators in balancing equations.
If you want to use this calculator, you must enter the chemical formula and compound in the following format.
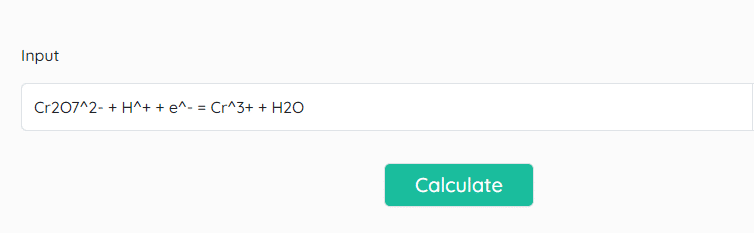
After the completion of entry in the correct format, click on “Calculate” and you can see the result as shown below.

Example 1: Simple Redox Reaction
Consider the equation:
Cu + 2AgNO3 -> Cu(NO3)2 + 2Ag
The oxidation half-reaction: Cu -> Cu2+ + 2e–
The reduction half-reaction: 2Ag+ + 2e– -> 2Ag
In the oxidation half-reaction:
Cu -> Cu2+ + 2e– (already balanced)
In the reduction half-reaction:
2Ag+ + 2e– -> 2Ag (already balanced)
In the oxidation half-reaction:
Cu -> Cu2+ + 2e– (no oxygen atoms)
In the reduction half-reaction:
2Ag+ + 2e– -> 2Ag + H2O
Conclusion
Balancing redox equations is a fundamental skill in chemistry, and the use of redox reaction calculators has revolutionized this process. These calculators streamline the balancing process, making it accessible to a wider audience and saving valuable time. By following the step-by-step guide outlined above, you can confidently balance redox equations with ease. Embrace the power of redox reaction calculators to enhance your understanding and mastery of redox chemistry.
FAQs
Can I use a redox reaction calculator for all types of redox reactions?
Yes, redox reaction calculators can handle various types of redox reactions, including simple and complex ones.
Are redox reaction calculators accurate in balancing equations?
Redox reaction calculators provide accurate results, but it’s crucial to double-check the balanced equation for precision.
Can I use a redox reaction calculator in exams or assignments?
It depends on the specific rules set by your instructor or exam guidelines. Ensure you are allowed to use calculators or technology during assessments.
Are redox reaction calculators free to use?
Many redox reaction calculators are available for free online, but some may offer additional premium features at a cost.
Are there limitations to using redox reaction calculators?
Redox reaction calculators are highly efficient, but they may struggle with extremely complex reactions or uncommon species. In such cases, manual balancing techniques may be necessary.