Table of Contents
ToggleA chemical equation is a symbolic expression of a chemical reaction. It is a shorthand representation that expresses a certain essential aspect of a chemical reaction by means of symbols and formulae. This sort of representation is called an equation since the number of atoms and mass of the reactant system may be equated with the number of atoms and mass of the product system, respectively. Thus, a chemical reaction is broadly defined as a brief representation of a chemical change in terms of symbols and formula of the species involved in a chemical reaction.
A chemical reaction between magnesium and sulphuric acid can be represented as:

The substances which are allowed to react themselves to bring about chemical reaction are known as reactants and the substances which are formed as a result of reactions are called products.
How to write a chemical equation?
The following points should be kept in mind while writing a chemical equation.
- The reacting species should be written on the left-hand side.
- If there are many reactants, a (+) sign is used to indicate their separation.
- A (+) sign separates each product from the others, which are arranged on the right-hand side.
- The reactants and products are separated by an arrow pointing towards the products.
- The species of the reactants and products should be numerically balanced.
For example, the reaction between silver nitrate and sodium chloride to form silver chloride and sodium nitrite.
AgNO3 + NaCl → AgCl + NaNO3
Here, the number of silver atoms on the left side = number of silver atoms on right = 1
Similarly, number of oxygen atoms on the left = number of oxygen atoms on right = 3
Likewise, the number of sodium, Nitrogen, and chloride in both the reactants and products are the same.
Essentials of a chemical equation
A chemical equation must satisfy the following conditions:
- It should be able to represent the actual chemical change.
- It should be balanced. This means that the total number of each kind of atom on both sides of the equation should be equal.
- It should be molecular i.e. all the elementary gases like hydrogen, nitrogen, oxygen, etc. must be presented in their chemical form.
Significance of a chemical equation
Chemical equations are convenient ways of representing the actual chemical reaction and give more information than the sentence description. A chemical equation has both qualitative and quantitative significance.
Qualitative significance: Helps to identify the reactants and the products.
Quantitative significance: Gives information about the reactants and products mathematically like:
- number of moles of reactants and products
- number of atoms or molecules taking part in the reactions.
- relative combining weight of reactants and products.
- volume of the gaseous species.
Let us take the following examples:
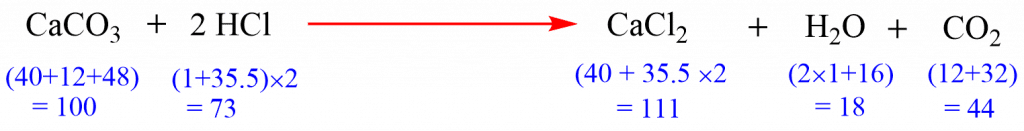
The above-mentioned equation gives qualitative information that CaCO3 reacts with HCl to produce CaCl2 and H2O.
This contrasts with the quantitative significance which tells that (i) 1 mole of CaCO3 reacts with 2 moles of HCl to form 1 mole of CaCl2, 1 mole of H2O, and 1 mole of CO2, (ii) 100 parts by weight of CaCO3 reacts with 73 parts by weight of HCl to give 111 parts by weight of CaCl2, 18 parts by weight of H2O, and 44 parts by weight of CO2, (iii) 22.4 liters of CO2 is produced at NTP when 1 mole of CaCO3 and 2 moles of HCl react with each other.
How to balance chemical equations?
A chemical equation must be balanced by adding the proper coefficients in front of the chemical symbols and formulas in order to follow the rule of conservation of mass. This actually means that mass is neither generated nor destroyed during a chemical process. In order to achieve this, the number of atoms on the reactant and product sides must be exactly equal. There are four methods of balancing the equations:
- Hit and trial method
- Partial equation method
- Oxidation number method
- Ion-electron method
Hit and trial method
This approach is also known as the trial-and-error approach. The simple chemical process can be balanced adequately with this method. It involves the following steps.
- The skeleton equation, which accurately reflects the reactants and products, is written.
- If elementary gases like hydrogen, oxygen, etc. are included in the equation, they are initially represented in their atomic form and are then transformed to their molecular form once the equation is balanced.
- First, the formula with the maximum number of elements is chosen, and its atoms are balanced.
- If the previous procedures don’t work, first the atoms of that element that are present in the fewest number of locations are balanced. Then, the atoms of the element with the greatest number of occurrences are balanced.
- Since each compound has a definite chemical formula, it is not appropriate to alter the chemical formula of any compound while balancing the chemical equation.
Let us consider the reaction of iron and water to form a magnetic oxide of iron and hydrogen.
The skeleton of the equation for the reaction is
Fe + H2O → Fe3O4 + H2
Elementary substance hydrogen is changed to atomic form.
Fe + H2O → Fe3O4 + H
To balance the number of atoms of LHS to RHS
3 Fe + 4 H2O → Fe3O4 + 8 H
8 atoms of H are written as 4 molecules in order to convert the equation into molecular form.
3 Fe + 4 H2O → Fe3O4 + 4 H2
This is the required balanced chemical equation.
Partial equation method
The partial equation method is more preferable than the hit and trial method. This approach is used in complex reactions where the same element might be found in multiple products. The partial equation method involves the following steps:
- For the given chemical equation, different possible steps are written. The chemical equations for probable steps are called partial equations.
- The hit and trial approach is used to individually balance each partial equation.
- The intermediate species are eliminated by multiplying each partial equation by the appropriate number (not involved in the final equation).
- The different partial equations are added to get the balanced equation.
The example depicting partial equation methods are:
P4 + HNO3 → H3PO4 + NO2 + H2O
The probable partial equations are:
2 HNO3 → 2NO2 + H2O + O …………………………(i)
P4 + 10 O → 2 P2O5 ……………………………………….(ii)
P2O5 + 3H2O → 2 H3PO4 …………………………….(iii)
Equation (i) is multiplied by 10 and equation (iii) is multiplied by 2:
Thus, the balanced partial equations are added so as to cancel nascent oxygen and P2O5, which are the intermediate products.
2 HNO3 → 2 NO2 + H2O + O ] ×10
P4 + 10 O → 2 P2O5
P2O5 + 3H2O → 2 H3PO4 ×2
P4 + 20 HNO3 → 4 H3PO4 + 20 NO2 + 4 H2O
This is the required balanced equation.
Limitations of a chemical equation
The chemical equation gives much useful information about chemical reactions. However, they have their limitations and do not tell us:
- The physical state of the reactants and products.
- The concentration of the reactant and product, both at the beginning and at the end.
- The speed at which the reaction proceeds and the time taken for the completion of the reaction.
- Reversible or Irreversible nature of the reaction.
- Endothermic or exothermic nature of the reaction.
- The condition under which the reaction takes place.
- The mechanism of the reaction which involves various intermediate steps engaged between the initial and final steps of the reaction.
The following corrections have been made to overcome the above-mentioned limitations so as to get the chemical equation representing the chemical reaction more authentic.
- Physical states of the species involved in reactions are represented by the letters (s), (l), or (g) for the solid, liquid, and gas respectively.
- Writing a brief description above or below the equality symbol or arrowhead is used to indicate conditions.
- The amount of heat can be written with a plus (+) or minus (–) sign at the side of the product to indicate evolution or absorption, respectively.
- Reversible reactions are shown by ⇌, while irreversible reactions are shown by → arrow.
- The equation does not explain the gases or precipitates produced in the reactions. Giving an upward arrow (↑) for the gas produced and a downward arrow (↓) for the precipitate formed helps to solve this problem.
Chemical equation Video
FAQs
What is a chemical equation?
A chemical reaction is broadly defined as a brief representation of a chemical change in terms of symbols and the formula of the species involved in a chemical reaction.






