Table of Contents
ToggleEthers are a class of compounds whose molecules have an oxygen atom bonded to two organic groups. The bivalent oxygen (-O-) is the functional group of ethers. Ethers are represented by the general formulae R-O-R, Ar-O-R, or Ar-O-Ar. Structurally, ethers may be considered to be derived from water by the replacement of both the hydrogens with alkyl or aryl groups.
Ethers may also be classified into two categories as aliphatic ethers and aromatic ethers. In aliphatic ethers, both the groups attached to ether oxygen are alkyl groups, whereas, in aromatic ethers, one or both groups are aryl groups.
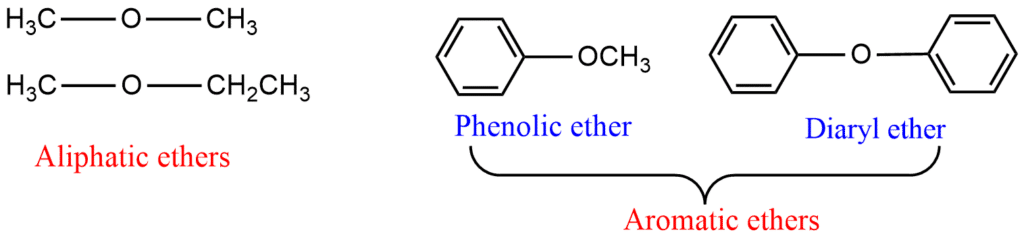
Nomenclature of Ethers
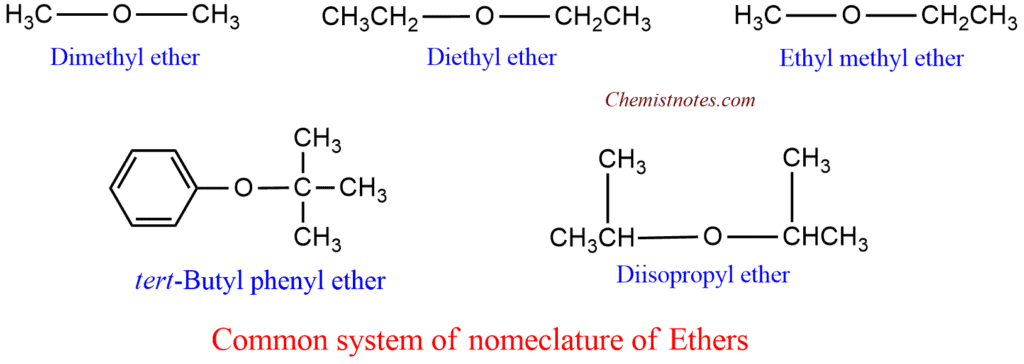
- Most of the ether are known by their common names which consist in writing the name of alkyl or aryl substituents in alphabetical order followed by the word ‘ether’. for example:
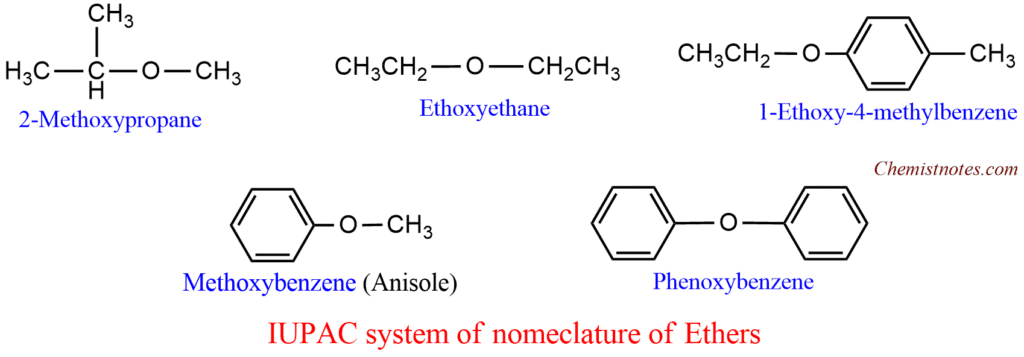
- In the IUPAC system, ethers are named as alkoxy derivatives of hydrocarbons. The smaller substituent along with oxygen is considered an alkoxy group (RO-). For example:
Preparation of Ethers
Ethers can be prepared by following general methods:
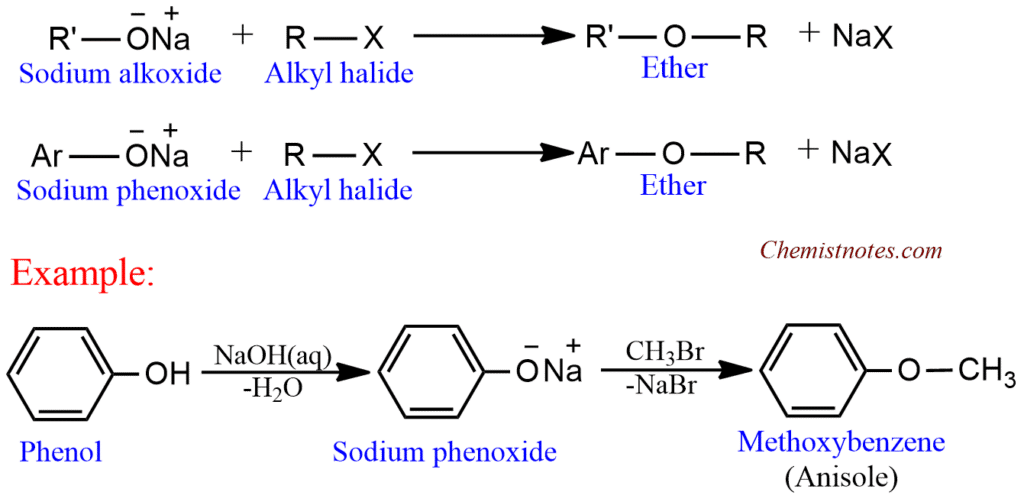
Williamson’s ether synthesis
This is the most important method for laboratory preparation of both symmetrical and unsymmetrical ether. It is a nucleophilic substitution reaction, consisting of treating appropriate alkyl halide with an alkoxide or phenoxide.
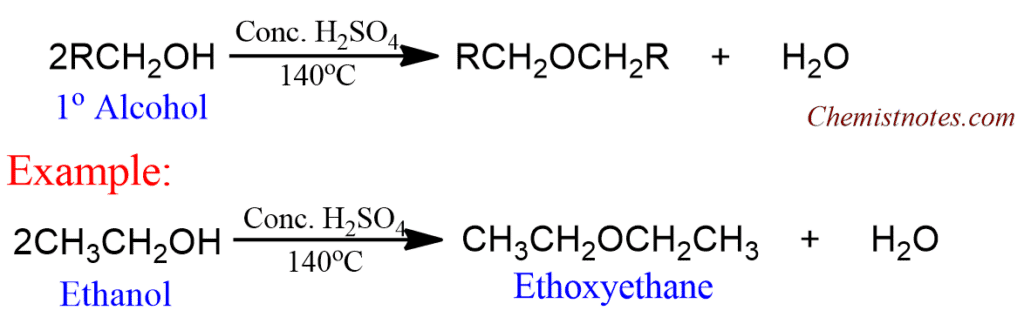
Dehydration of alcohol
This method is used to prepare symmetrical ethers with primary alkyl groups. In this method, excess of a primary alcohol is heated with conc. H2SO4 at 140oC to get symmetrical ether.
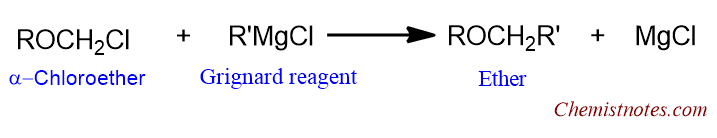
Use of Grignard reagent
This method is used to prepare higher ethers. In this method, a halogen-substituted lower ether is treated with a suitable Grignard reagent to get higher ether.
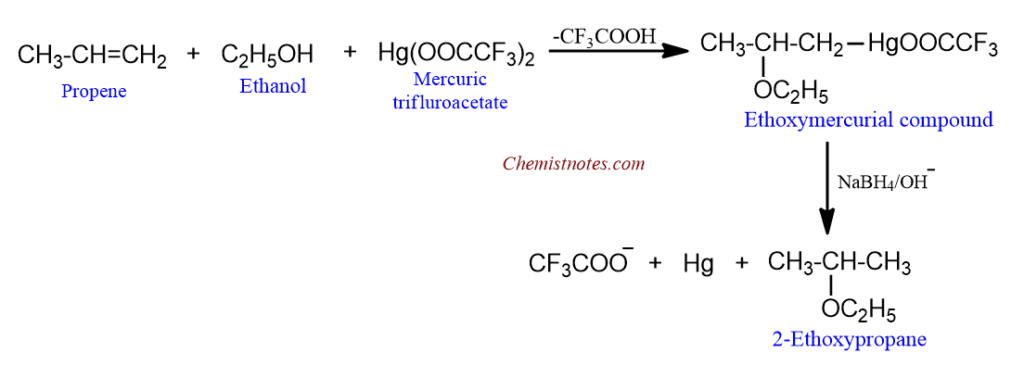
Alkoxymercuration-demercuration of alkenes
Alkenes readily react with mercuric trifluoroacetate in the presence of an alcohol to give alkoxy mercurial compounds which upon reduction with NaBH4 in basic medium gove ethers in high yield.
Properties of Ethers
Physical Properties
- Lower members (dimethyl ether, ethyl methyl ether) are colorless gases, and higher members are low-boiling colorless liquids with a pleasant smell.
- Ethers have a similar structure to that of water (H2O), but the bond angle in ether is about 110o, which is slightly greater than that of water molecules (104.5o).
- Ethers have bent or angular structures and therefore, ethers have a net dipole moment.
- Ethers have much lower boiling points as compared to isomeric alcohols but their boiling point is close to alkanes of comparable molecular weights.
- The solubility of ethers is comparable to those of corresponding alcohols. However, the solubility of ethers in water decreases from lower members to higher members.
| Ethers | Structure | Dipole moment (D) | B.P. (oC) | M.P. (oC) | Solubility in 1 liter of H2O |
| Dimethyl ether | CH3-O-CH3 | 1.3 | -23.0 | -138.5 | 70g |
| Diethyl ether | CH3CH2-O-CH2CH3 | 1.18 | 35 | -116.3 | 69g |
| Tetrahydrofuran | (CH2)4O | 1.74 | 66 | -108.4 | miscible |
| Dioxane | O(C2H4)2O | 0.45 | 101.1 | 11.8 | miscible |
Chemical Properties
Ethers are relatively inert compounds in spite of the presence of oxygen atoms carrying two lone pairs of electrons in their molecules. Ethers are not easily attacked by alkalies, dilute acids, metallic sodium, etc. under ordinary conditions. It is because of this reason that these are used as solvents. They undergo chemical reactions under specific conditions.
- Ethers on exposure to air and light undergo slow oxidation to form a mixture of hydroperoxide and dialkyl peroxide.
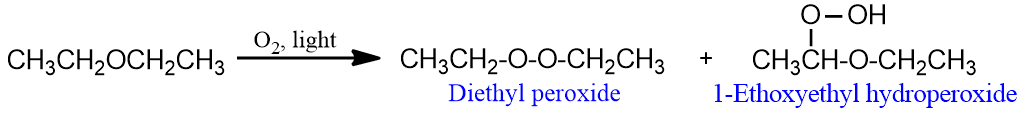
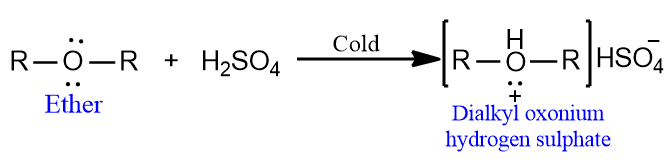
- Ethers act as Lewis bases due to the presence of lone pairs of electrons on oxygen atoms and dissolve in cold concentrated mineral acids (H2SO4 and HCl) to form salts called oxonium salts.
- Ethers can be cleaved by the use of hydriodic acid or hydrobromic acid to give alkyl halides and alcohol.
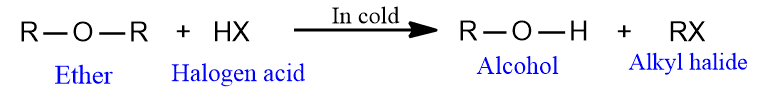
- Ethers react with chlorine or bromine to give substituted products. The extent of substitution depends upon the reaction conditions.
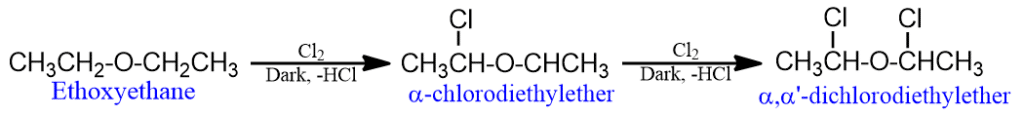
- A mixture of vapors of ether and air is explosive and burns with a luminous flame.
- When ether vapours are passed over heated alumina at about 380o, dehydration takes place and alkenes are formed.
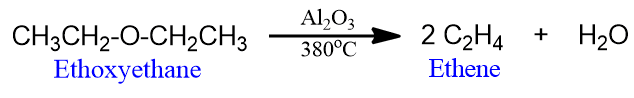
Uses of Ethers
- Ethers are used as solvents both in the laboratory and in industry.
- Lower ethers are used as general anesthetics. Since they produce intense local cooling when sprayed on the skin, ethers are also used as local anesthetics for minor surgical operations.
- The lower ethers are volatile liquids that on evaporation produce low temperatures. They are, therefore, used as refrigerants.
- Polyethylene glycol(PEG) is a linear polyether used in cosmetics and pharmaceuticals.
- Crown ethers are cyclic polyethers that are used as phase transfer catalysts.
FAQs
What is an ether?
Ethers are a class of compounds whose molecules have an oxygen atom bonded to two organic groups.
What does ether smell like?
Ethers smell sweet and mildly pungent.
Is diethyl ether polar?
Diethyl ether is a non-polar solvent.
What is ether used for?
Different types of ether are used for different purposes, Lower ethers are used as general anesthetics, and some ethers are used in cosmetics also.






