Table of Contents
ToggleWhat is Solute?
A solute is a substance that can be dissolved by a solvent to the formation of a solution. When it comes into contact with the appropriate type of solvent, it starts the dissolution process. During the dissolution process, the solvent separates the molecules of solute and disperses them uniformly. This generates a homogenous mixture which we refer to as a solution.
Characteristics of Solute
- The solute can exist in all three states of matter: solid, liquid, or gas.
- The solute completely dissolves in another substance in a homogeneous mixture, and the solute is uniformly distributed throughout the solution.
- The solute is not distributed uniformly in a heterogeneous mixture, and its concentration varies across the solution.
- The boiling point of a solute is greater than that of a solvent.
- A solution will always contain a lower concentration of the solute than the solvent.
- Heat is transferred to the solute in a solution.
- The ability of a solute to solubilize is also determined by its chemical structure and characteristics. A polar solute, for example, will dissolve easily in a polar solvent, and vice versa.
- As the surface area of the solute particle increases, it also increases its solubility.
- Aside from that, the volume and temperature also influence the level of dissolution.
- In the case of gaseous solutes, pressure also affects solubility besides volume and temperature.
Examples of solute
- Sodium chloride (NaCl) and other salts
- Dyes
- Sugar
- Carbon dioxide in sodas
- Cocoa in hot chocolate
What is a Solvent?
The solvent is a substance in which solute dissolves during the formation of the solution. It can break the molecular interaction between solute-solute molecules and suspend the free solute molecules evenly to make a solution. The solvent occupies the majority of the solution since its amount is always greater than that of the solute.
Characteristics of Solvent
- Solvents are typically liquids, although they can also be solids or gases.
- The solvent breaks the larger solute particle into smaller particles, which are then dispersed throughout the solution.
- Solvent has a low boiling point and evaporates easily.
- Organic solvents contain carbon, whereas inorganic solvents are without carbon.
- In a solution, heat is transferred from the solvent.
- All solvent proportions will have the same solute concentration.
- Solvents are also used to control the temperature of a solution, either by absorbing the heat created during a chemical reaction or by increasing the rate of the reaction with the solute.
Types of solvents
- Hydrocarbon solvents: Hydrocarbon solvents are organic solvents that contain only hydrogen and carbon atoms. Hydrocarbon solvents are formed as volatile fractions in crude oil refineries. The resulting hydrocarbon solvents contain varying amounts of paraffinic, naphthenic, and aromatic components.
- Oxygenated solvents: Oxygenated solvents are those that contain carbon, hydrogen, and oxygen atoms in their chemical structure. The oxygen molecule exists in an oxygenated solvent with high solvency and low toxicity. These solvents are used in paints, inks, pharmaceuticals, perfumes, adhesives, cosmetics, detergents, and food processing.
- Halogenated solvents: Halogenated solvents are those that contain a halogen, such as chlorine, bromine, or iodine. Many people are familiar with perchloroethylene, a highly effective solvent used in dry cleaning.
Examples of Solvent
- Water
- Oil
- Isopropyl alcohol
- Acetone
- Acetic Acid
- Ethanol
- Chloroform
- Toluene
Difference Between Solute and Solvent
| Comparison element | Solute | Solvent |
| Definition | A solute is a substance that dissolves with solvent to form a solution. | The solvent is a substance in which solute dissolves during the formation of the solution. the solvent is usually a liquid. |
| Phase | A solute is the dispersed phase of the solution. | The solvent is the solution’s medium phase, which disperses the solute particles. |
| Quantity | The amount of solute in a solution is less than the amount of solvent. | The amount of solvent is more than the solute in a solution. |
| Physical state | It can exist as a gas, liquid, or solid. | The solvent is usually a liquid, but some solvents might exist in the gaseous state. |
| Boiling point | The boiling point of solute is higher than that of solvent. | The boiling point of solvent is less than that of solute. |
| Solubility | The solute’s solubility is determined by properties such as surface area and molecule size. | Solubility is determined by the solvent’s properties, such as polarity. |
| Heat transfer | Heat is transferred to the solute in a solution. | In a solution, heat is transferred from the solvent. |
| Examples | Examples of solutes are sugar, dissolved carbon dioxide, oxygen, water vapor, carbon dioxide, argon | Examples of solvents are water, Ethanol, Methanol, Acetone, tetrachloroethylene, Toluene, Methyl acetate, and Ethyl acetate. |
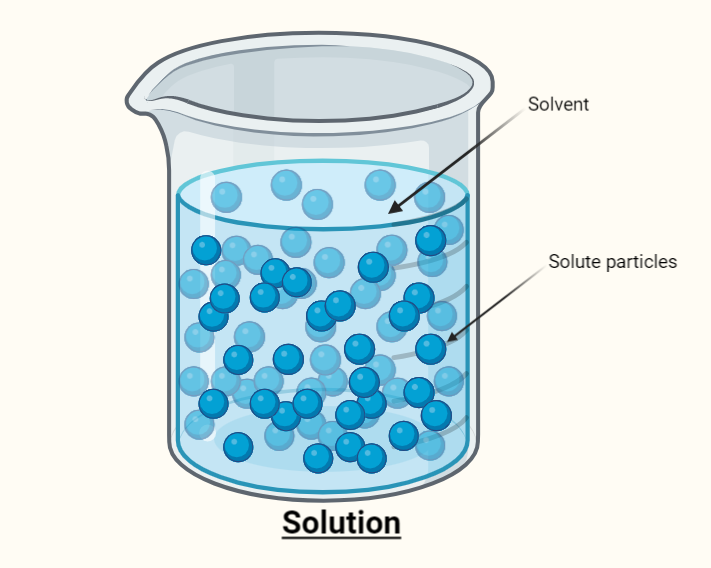
Solute in the solvent
A solution is made up of only two components i.e., solute and solvent.
For examples;
- Salt in seawater
- Ethylene glycol in water
- Sugar in coffee or tea
- Brass (various metals mixed into copper)
Related video
FAQs
Is water solute or solvent?
Water is also known as the “universal solvent” because it can dissolve almost any material better than any other liquid.
What is the solution?
A solution is a mixture of solute and solvent.
What are organic solvents made of?
Organic solvents are carbon-based solvents (i.e., they contain carbon in their molecular structure).






