Table of Contents
ToggleSome of the general methods for the preparation of alcohol are discussed below:
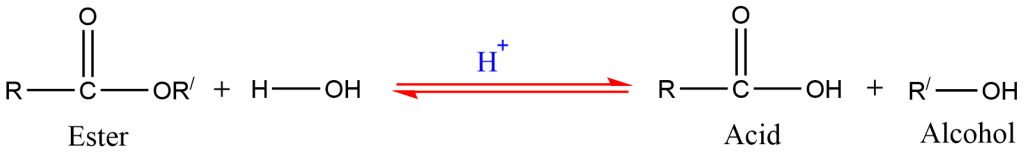
1. Hydrolysis of ester
Alcohols are prepared by the hydrolysis of ester in the presence of acid.
2. Hydrolysis of alkyl halides
Hydrolysis of alkyl halides with aqueous sodium or potassium hydroxide gives alcohol.
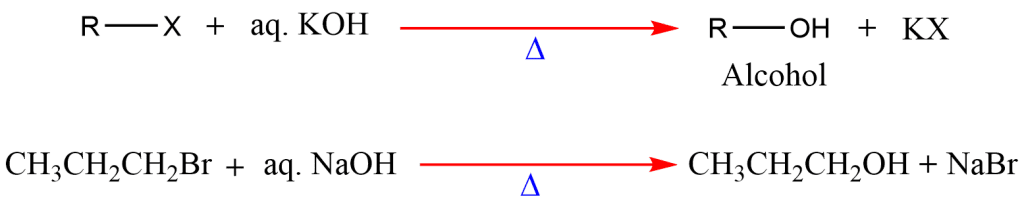
It is not a particularly effective method of preparing alcohols because the tertiary alkyl halides undergo a competitive elimination that results in the undesirable side reaction yielding alkene.
3. From Primary amines
Aliphatic primary amines in reaction with nitrous acid (NaNO2 + HCl) at 0oC-10oC temperature give alcohol.
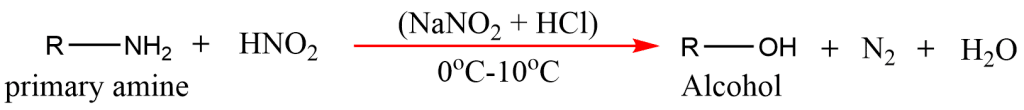
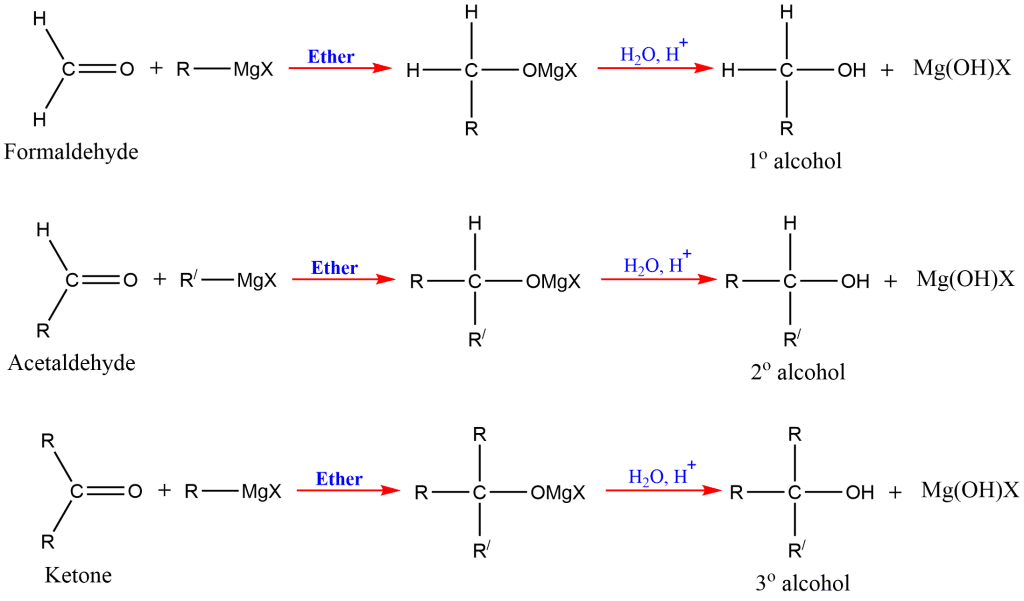
4. Grignard Synthesis
This is one of the most important methods for the preparation of different orders of alcohols; primary, secondary, and tertiary. The alkyl or aryl magnesium halides are commonly known as Grignard reagents.
In the presence of dry ether, the Grignard reagent reacts with a carbonyl molecule (aldehyde or ketone) to produce an addition product that, upon acidic hydrolysis, yields the corresponding alcohol ( 1o, 2o and 3o).
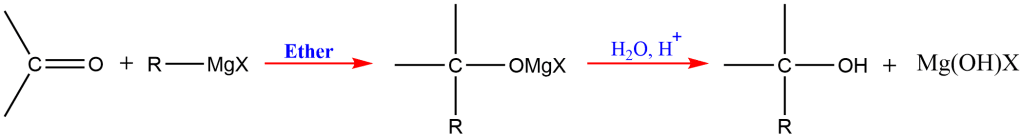
Reaction of Grignard reagents with formaldehyde gives primary alcohols, while the aldehydes other than formaldehyde give secondary alcohol. Likewise, Ketones give tertiary alcohol in reaction with this reagent.
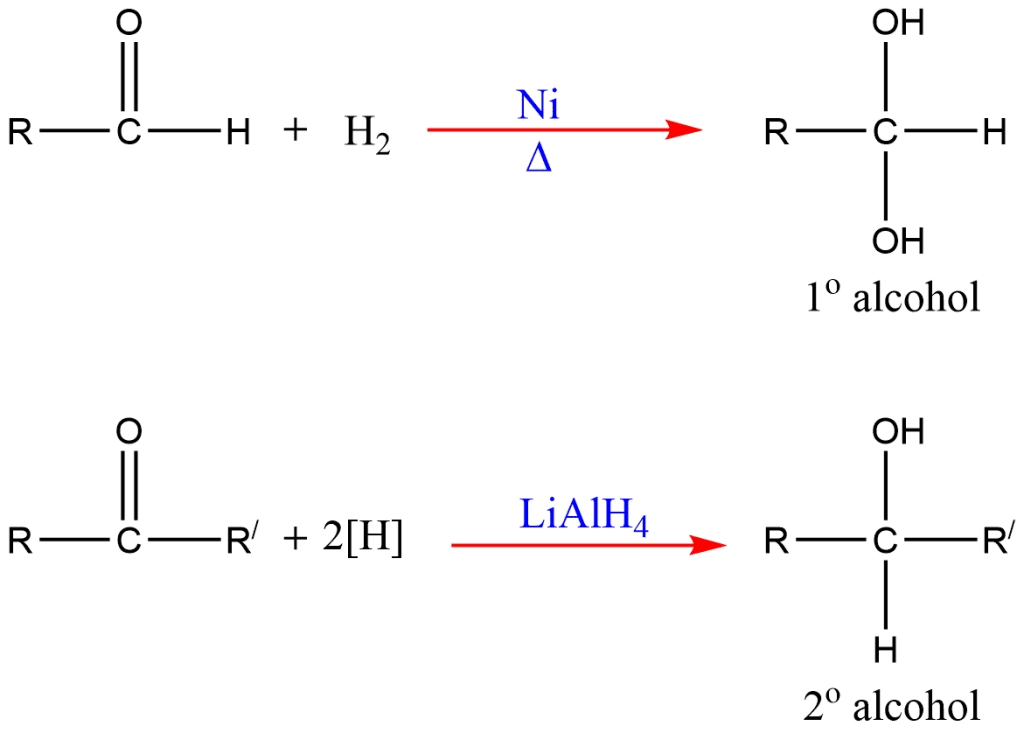
5. Reduction of carbonyl compounds
Carbonyl compounds (aldehyde and ketone) on reduction with H2/Ni or LiAlH4 (Lithium aluminum hydride) give corresponding alcohols.
On reduction, aldehyde gives primary alcohols and ketone gives secondary alcohols.
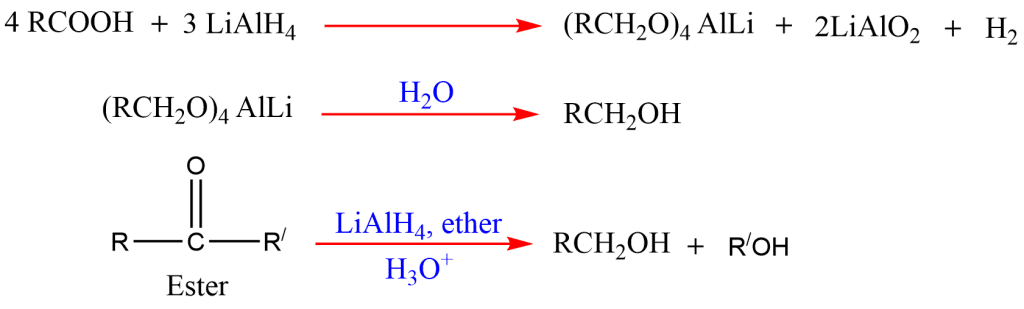
6. Reduction of acid and esters
Alcohol can be prepared by the reduction of carboxylic acids and esters with reducing agents, lithium aluminum hydride.
7. Oxymercuration-Demercuration of alkene
It is one of the most important methods for the preparation of alcohol. Alkene reacts with mercuric acetate in a mixture of tetrahydrofuran (THF) and water to form hydroxy alkyl mercuric compounds, which on reduction with sodium borohydride yields alcohol. The mechanism involves markonikov’s rule of addition of water to Carbon-carbon diuble bond.

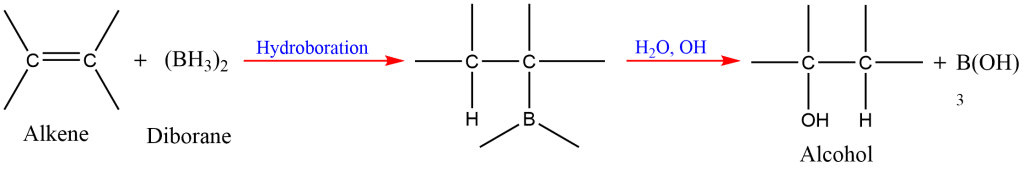
8. Hydroboration-Oxidation of Alkene
Alkene reacts with diborane to form trialkylboranes, which on oxidation with alkaline hydrogen peroxide form alcohols.
Note: Click on this to learn more about the mechanism of Hydroboration-oxidation of alkene.
9. Aldol Condensation
In the presence of a base, two molecules of an acetaldehyde or ketone containing α-hydrogens combine to form a β-hydroxyaldehyde or β-hydroxyketone.
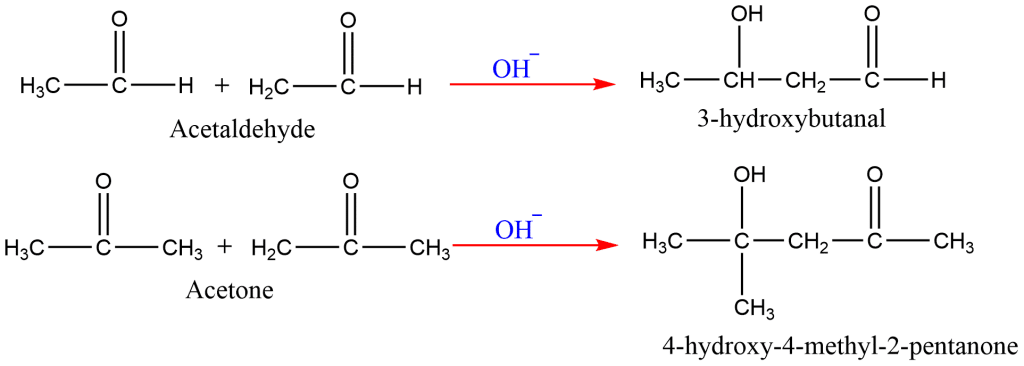
Note: Please click on this to learn the mechanism of aldol condensation
Preparation of alcohol video
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.






