Table of Contents
ToggleSiegmund Gabriel was the first to report this reaction in 1887, so it is known as the Gabriel synthesis. This reaction is also known as Gabriel primary amine synthesis or Gabriel phthalimide synthesis.
Other methods of preparing amines, such as alkylation or ammonia, produce not only primary amines but also secondary and tertiary amines. To avoid the formation of secondary and tertiary amines, Gabriel synthesis is one of the best methods for the preparation of primary amines only.
How to prepare Potassium phthalimide?
The main reagent in Gabriel synthesis is Potassium phthalimide, which can be prepared by reacting phthalimide with potassium hydroxide.
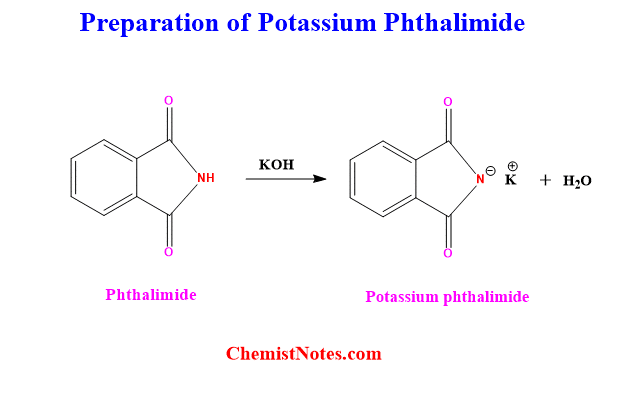
Is this proton attached to the N-atom acidic? Yes, it is acidic because it is flanked by two C=0 groups, just like in beta-keto ester.
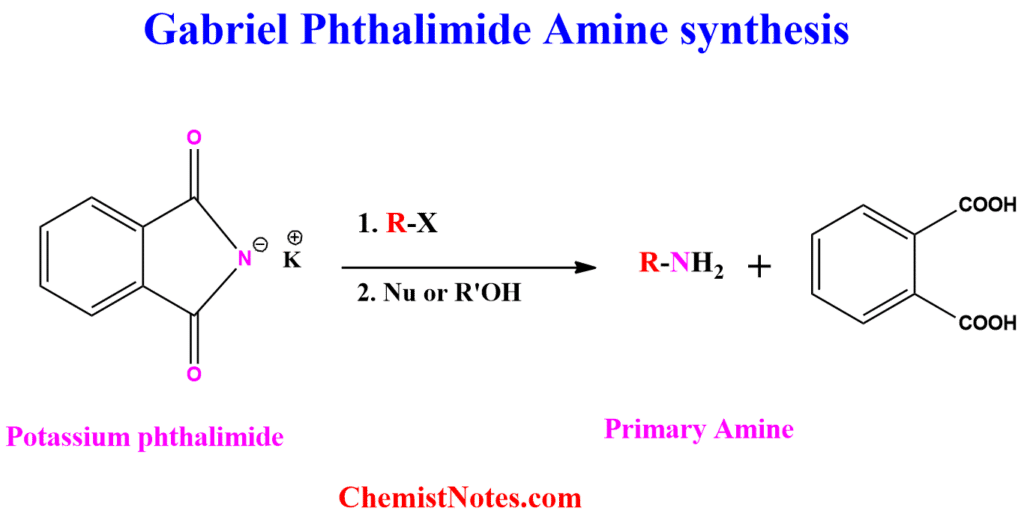
Here, potassium phthalimide acts as a nucleophile, and hence it attacks the alkyl halide via SN2 reactions. Therefore, this reaction works best for primary alkyl halides. Sometimes secondary alkyl halides are used, but not tertiary alkyl halides.
Gabriel synthesis
Gabriel synthesis involves the synthesis of Primary amines using potassium phthalimide and alkyl halides. The process consists of two sequential steps to synthesize primary aliphatic amines: first, the N-alkylation of phthalimide, and then the subsequent acidic or basic hydrolysis of N-alkyl phthalimide.
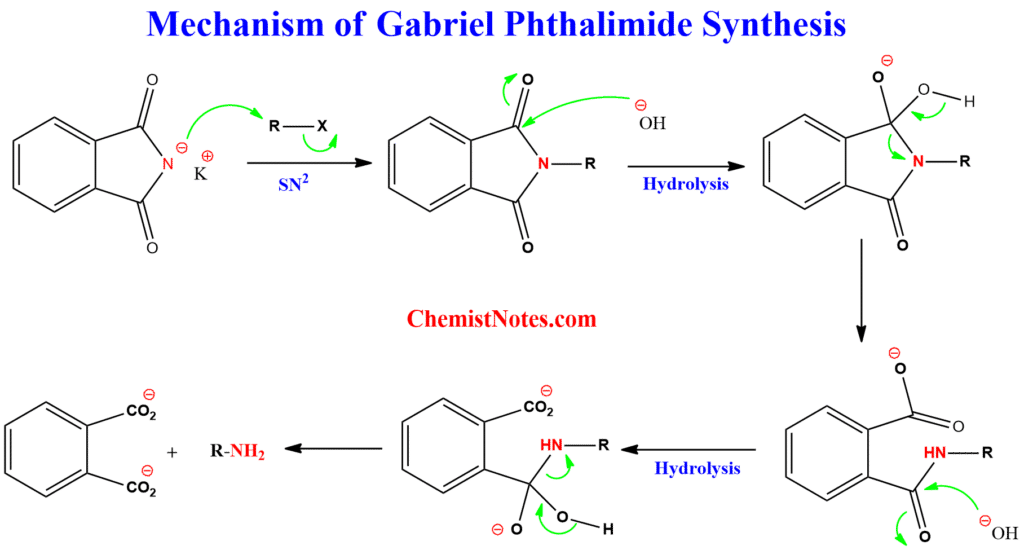
Gabriel synthesis mechanism
In this reaction, the presence of two electron-withdrawing carbonyl groups in phthalimide makes it readily deprotonate by KOH or NaOH, making it an excellent nucleophile.
The mechanism of Gabriel amine synthesis is a two-step process, as shown below:
Step 1: The phthalimide attacks the primary alkyl halide via the SN2 mechanism, forming N-alkyl phthalimides.
Step 2: The N-alkyl phthalimides are then hydrolyzed either in an acidic or basic workup. However, acidic hydrolysis is generally applied.
Gabriel synthesis procedure
The procedures to carry out Gabriel synthesis are listed below:
- First of all, dissolve phthalimide in hot water to prepare a solution. Take a small amount of hot water.
- Add potassium hydroxide solution to the phthalimide solution until the solution becomes basic.
- The solution is cooled so that a white precipitate is formed, which is filtered and dried.
- The white precipitate of potassium phthalimide is then dissolved in ethanol.
- After that, a primary alkyl halide is added to the above ethanol solution, followed by a KOH solution.
- The whole solution is kept in an ice bath for 15-20 minutes.
- Furthermore, the mixture is then poured into the diluted HCl solution and heated gently to form primary amine
- Finally, primary amine is extracted using a solvent extraction process.
Note: Alkyl azide is formed as an intermediate in this reaction, which is explosive in nature. Therefore, special care and safety should be taken.
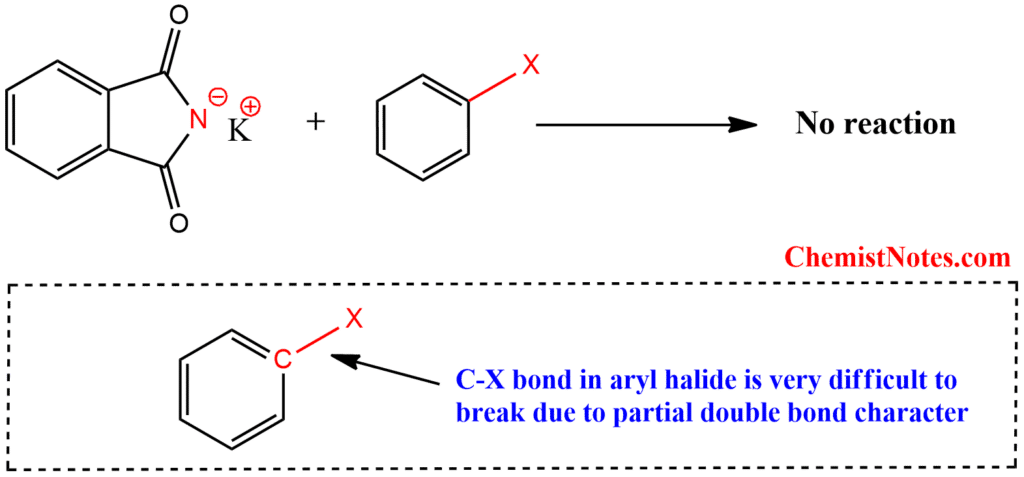
Why can’t aromatic primary amines be prepared by Gabriel phthalimide synthesis?
The C-X bond in aryl halides is challenging to break due to its partial double bond character. Moreover, aryl halides do not readily undergo nucleophilic substitution with the anion formed by phthalimide. As a result, it is not feasible to prepare aromatic primary amines by Gabriel phthalimide synthesis.
Application of Gabriel synthesis
Gabriel synthesis has wider applications in various fields, including organic synthetic chemistry, pharmaceuticals, polymer chemistry, etc. The main application in synthetic chemistry is the preparation of primary amines. For example: Amphetamine, a psychostimulant drug, can also be prepared by using Gabriel synthesis.
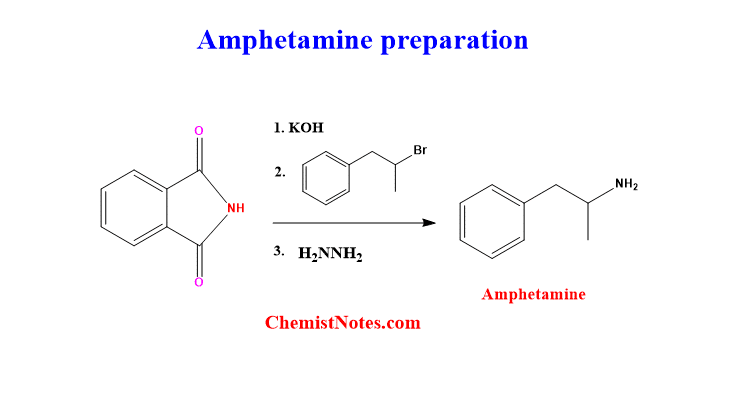
Moreover, Gabriel’s reaction can be used for the synthesis of amino acids as shown below.
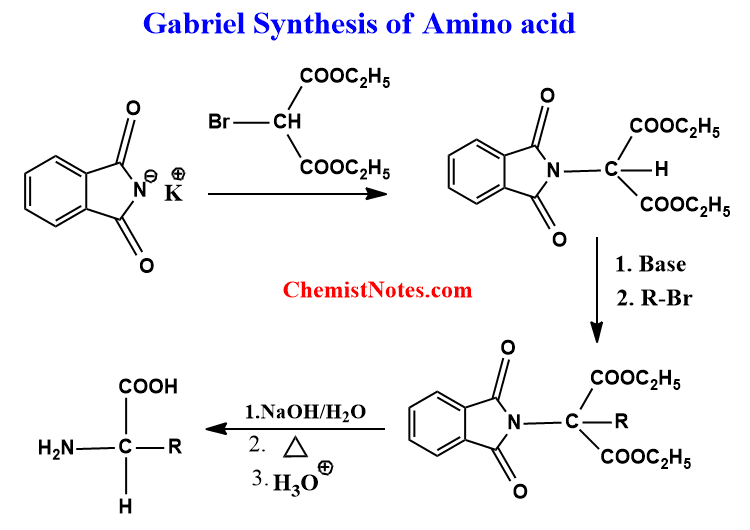
Advantages of Gabriel synthesis
The major advantage of this reaction is to avoid over-alkylation on the nitrogen atom so that only primary amines are formed.
Limitations of Gabriel synthesis
Only primary amines can be synthesized by this reaction, we can not prepare secondary and tertiary amines. This is the major limitation of this synthetic method. Moreover, aromatic primary amines can not be produced by Gabriel synthesis.
FAQs/MCQs
Are Strecker and Gabriel syntheses most important for mCAT?
Yes, Strecker and Gabriel syntheses are most important for mCAT.
Does a Gabriel synthesis require a primary alkyl halide?
Yes, Gabriel synthesis requires a primary alkyl halide. Secondary and tertiary alkyl halides can not undergo reaction in this method.
Does Gabriel synthesis produce a racemic mixture?
Yes, Gabriel synthesis produces a racemic mixture because the intermediate alkyl azides can attack phthalimide from either side.
Which primary amine cannot be prepared using Gabriel synthesis?
Aromatic primary amines can not be prepared using Gabriel synthesis.
What type of reaction occurs in Gabriel synthesis?
A nucleophilic substitution (SN2) reaction occurs in the Gabriel reaction.
Who was the first to report Gabriel phthalamide amine synthesis?
Siegmund Gabriel was the first to report this reaction.






