Table of Contents
ToggleAcid and bases have been defined in a variety of ways, each corresponding to a distinct approach to the properties of acidity and basicity.
Arrhenius theory
Acids were recognized in the early stages of chemistry by their sour taste and action on some plant pigments such as litmus. Bases were chemicals that interacted with acids to generate salts. Water was used almost exclusively for reaction in solution and in 1884 Arrhenius suggested the theory of electrolytic dissociation and proposed the self-ionization of water.
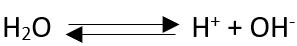
Thus according to Arrhenius” An acid is any hydrogen containing compound which gives H+ ions in solution.” for example:
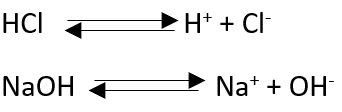
The concept is useful only in aqueous solution, if it is in organic solution or when it is in gaseous form or if it is in non-aqueous solvents.
Bronsted-Lowery theory
In 1923, JN Bronsted and T.M lowery independently suggested that, the strength of an acid depends upon its tendency to give up a proton, and the strength of a base depends upon its tendency to accept a proton. Sulfuric acid and hydrogen chloride are strong acids since they tend to give up a proton very readily; conversely, bisulfate ion, HSO4–, and chloride ion must necessarily be weak bases since they have little tendency to hold on to proton.
For aqueous solutions, the Bronsted-Lowery definition doesn’t differ appreciably from the Arrhenius definition of hydrogen ions (acids) and hydroxide ions (bases).
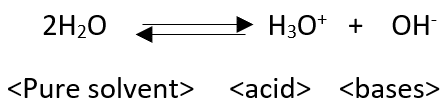
The usefulness of the Bronsted-lowery definition lies in the ability to handle any protonic solvent such as liquid ammonia or sulphuric acid.
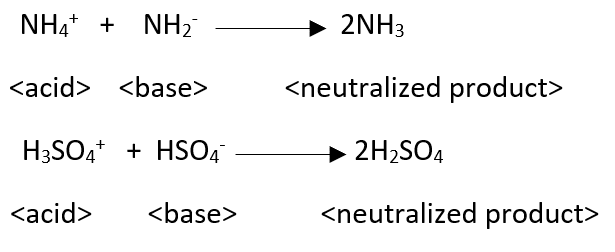
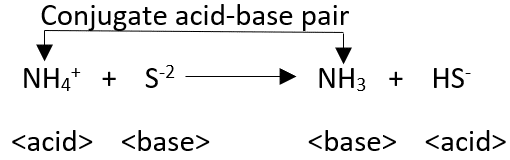
“Chemical species that differ from each other only to the extent of the transferred proton are termed conjugates.” Bronsted Lowery concept is not suitable for non-protonic systems (liq.SO2, N2O4, AlCl3, BF3, etc).
Lewis Acids and Bases
At about the same time that Bronsted proposed his acid-base theory, Lewis put forth a broader theory. A base in the Lewis theory is the same as in the Bronsted one, namely, a compound with an available pair of electrons, either unshared or in a π orbital. However, a Lewis base donates electrons to an atom other than H or C. A Lewis acid is any species with a vacant orbital. Thus, an acid is an electron-pair acceptor and a base is an electron-pair donor. In a Lewis acid-base reaction, the unshared pair of the base forms a covalent bond with the vacant orbital of the acid.
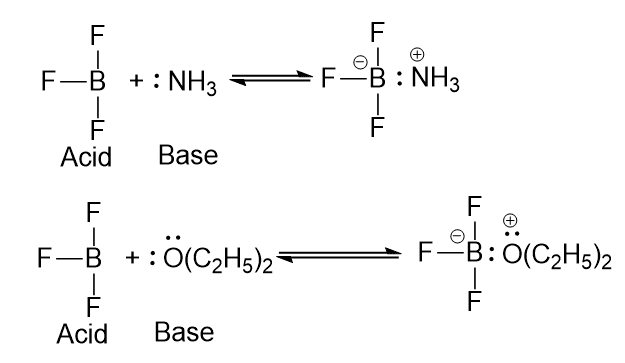
A proton is an acid because it is deficient in electrons, and needs an electron pair to complete its valance shell. Hydroxide ions, ammonia, and water are bases because they contain electron pairs available for sharing. In boron trifluoride, BF3, boron has only six electrons in its outer shell and hence tends to accept another pair to complete its octet. Boron trifluoride is acid and combines with such bases as ammonia or diethyl ether.
FAQs
Is NaOH an acid or base?
NaOH is the base.
Is HNO3 an acid or base?
HNO3 is an acid.
Is ammonia acid or base?
Ammonia is the base.
Is HCl an acid or base?
HCl is an acid.
Is HCN an acid or base?
HCN IS an acid.
Is vinegar acidic or basic?
Vinegar is a weak acid.
KOH an acid or base?
KOH is a base.
Baking soda is an acid or base?
Baking soda is a base.
Bleach an acid or base?
Bleach is an acid.
Is milk an acid or base?
Milk is an acid because of presence of lactic acid.
Is H2SO4 an acid or base
H2SO4 is an acid.






