Table of Contents
ToggleWhat is Gilman reagent?
Gilman reagent is an organometallic reagent containing lithium (Li), copper (Cu), and two alkyl or aryl groups. The Gilman reagent is also known as lithium dialkylcuprate or lithium diarylcuprate and is represented by R2CuLi. The Gilman reagent reacts with alkyl bromides, chlorides, and iodides in ether or tetrahydrofuran (THF) as a solvent to give a significant amount of cross-coupling products.

Preparation of Gilman reagent
Generally, Gilman reagent is prepared in two steps:
- In the first step, alkyl halide (R-X) is treated with lithium (Li) in the presence of dry ether to get alkyl lithium (R-Li).
- In the second step, the alkyl lithium (R-Li) is treated with cuprous iodide (CuI) in tetrahydrofuran (THF) solvent at -78 °C to get lithium dialkyl cuprates (Gilman reagent).
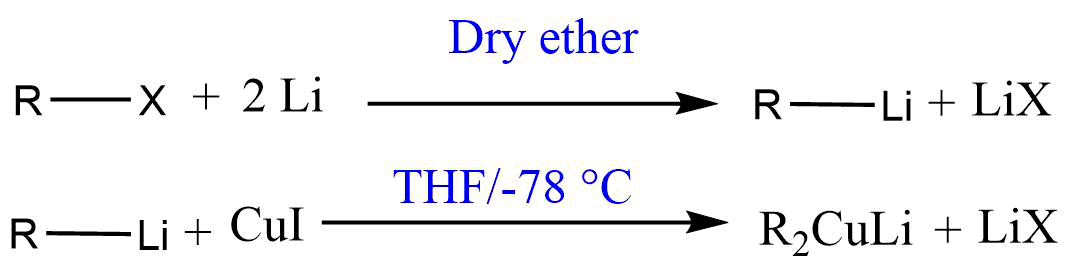
Gilman reagent is usually prepared at a temperature below 0 °C due to the thermal instability of the dialkyl cuprate containing hydrogen atoms on the carbon beta to Cu.
Structure of Gilman Reagent
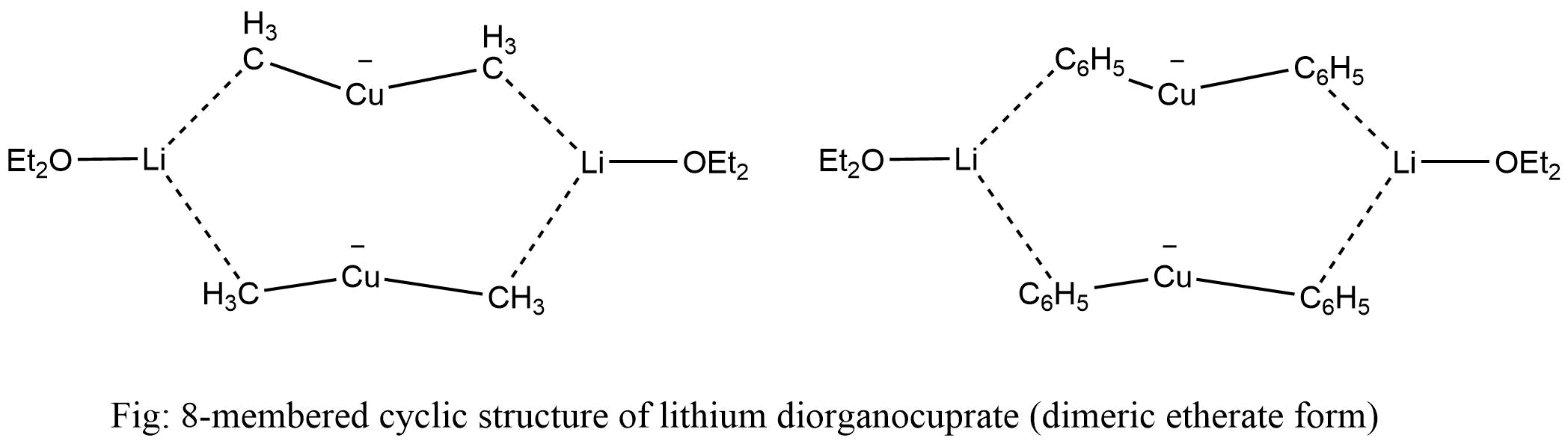
In diethyl ether solution, lithium dimethyl cuprate exists as a dimer, forming an 8-membered cyclic structure. Likewise, lithium diarylcuprate also crystallizes as dimer etherate.
But, if the Li+ ion forms a complex with crown ether like 12-crown-4, the diorganocuprate anion exists in a linear structure.
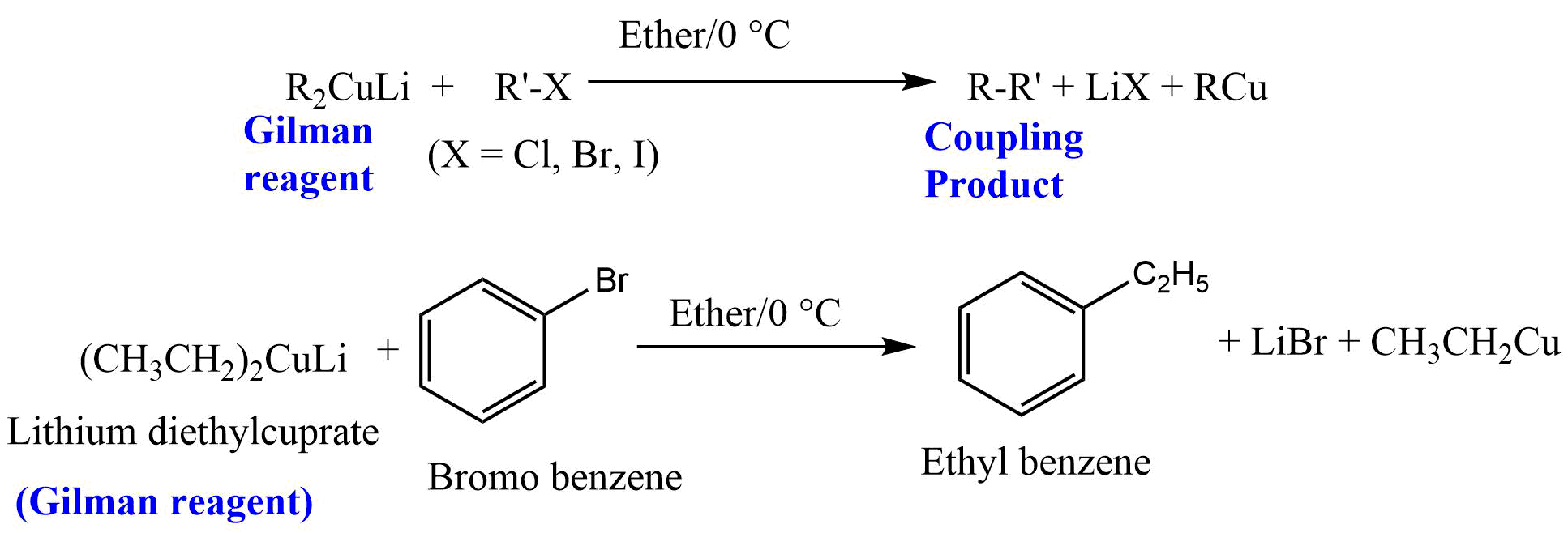
Coupling Reaction with Gilman Reagent
The coupling reaction of Gilman reagent with alkyl halides is of wide scope and R in R2CuLi may be primary alkyl, allylic, benzylic, aryl, vinyl, or allenic and may contain keto, COOH, COOR, or CONR2 group.
Gilman Reagent Mechanism
The mechanism of the coupling reaction probably involves the formation of Cu(III) intermediate and proceeds in two steps. In the first step, the Gilman reagent acts as a nucleophile, and the copper atom of the Gilman reagent undergoes an SN2-type substitution reaction with an alkyl halide to give Cu(III) intermediate. Thus, the formed neutral tricopper intermediate undergoes reductive elimination to give the coupling product. During the reductive elimination, the copper (III) complex is reduced to Cu(I). The general reaction mechanism of the Gilman reaction is outlined below:
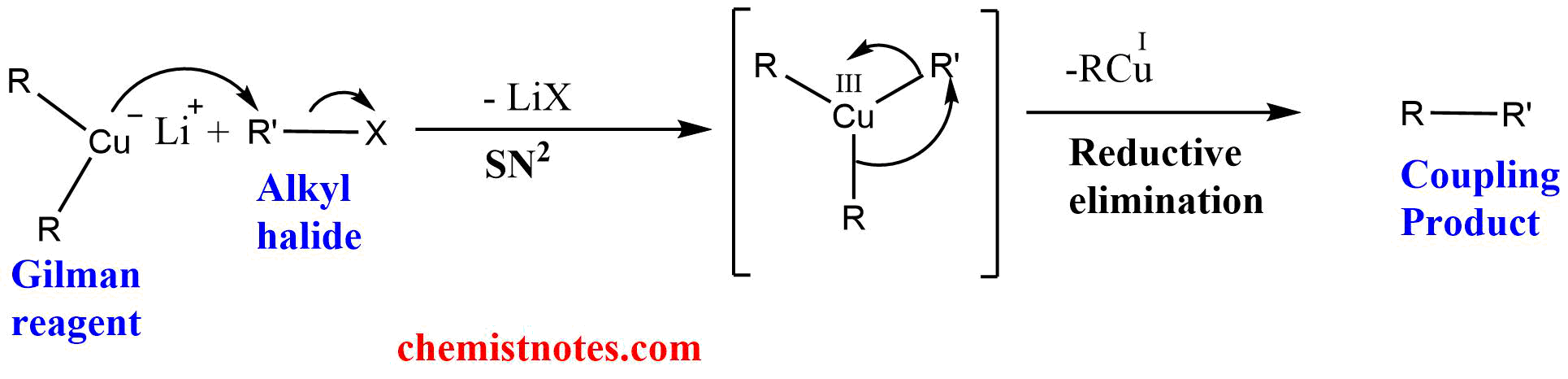
Stereochemistry of Gilman Reagent
The coupling reaction with allylic substrates usually proceeds via an SN2-type reaction with high selectivity for the γ-position.
The coupling reaction of Gilman reagent (Ph2CuLi) with 2-bromobutane proceeds via retention of configuration but the same reaction with 2-iodobutane proceeds via racemization.
The reaction at a vinylic substrate is stereospecific and usually proceeds with the retention of configuration.
Generally, gem-dihalides do not react with the Gilman reagent, but when two halogens are present on the carbon α to an aromatic ring or on a cyclopropane ring both halogens can be replaced by R. However, 1,2-dibromides exclusively give an elimination product.
Application of Gilman Reagent
Gilman reagent is widely used for the preparation of new compounds having carbon-carbon bonds from alkyl, aryl, acyl, vinyl, and allyl halides and also from α,β-unsaturated ketones.
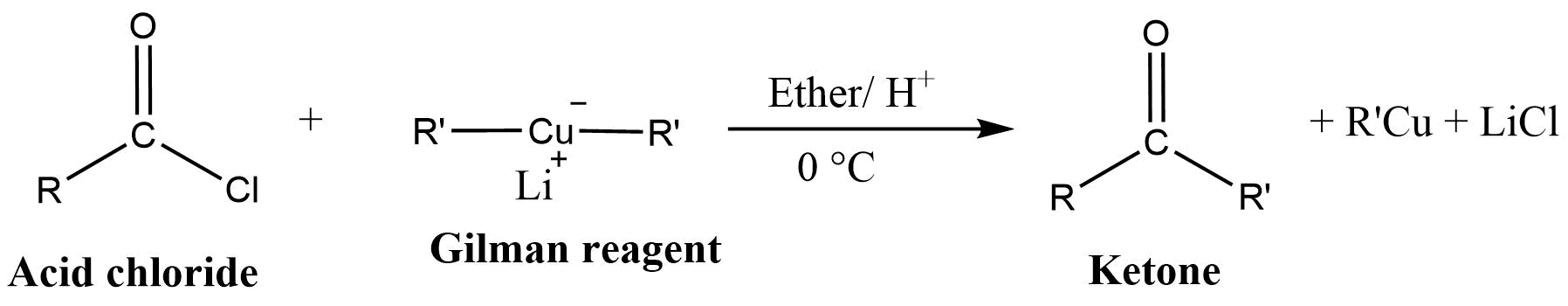
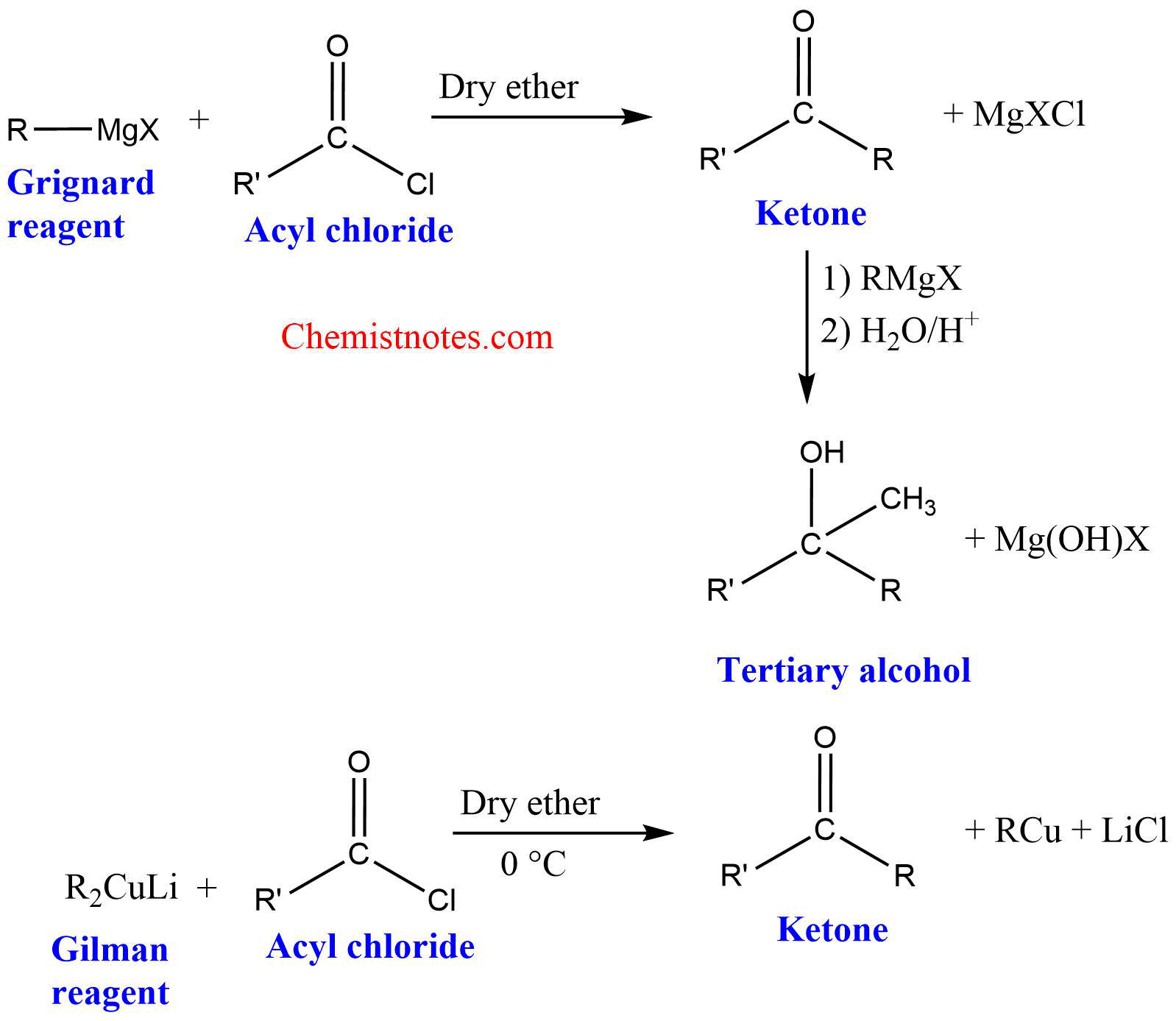
Reaction of Gilman reagent with acid chloride
Gilman reagent (lithium dialkylcuprate) reacts with acid chlorides to produce ketones.
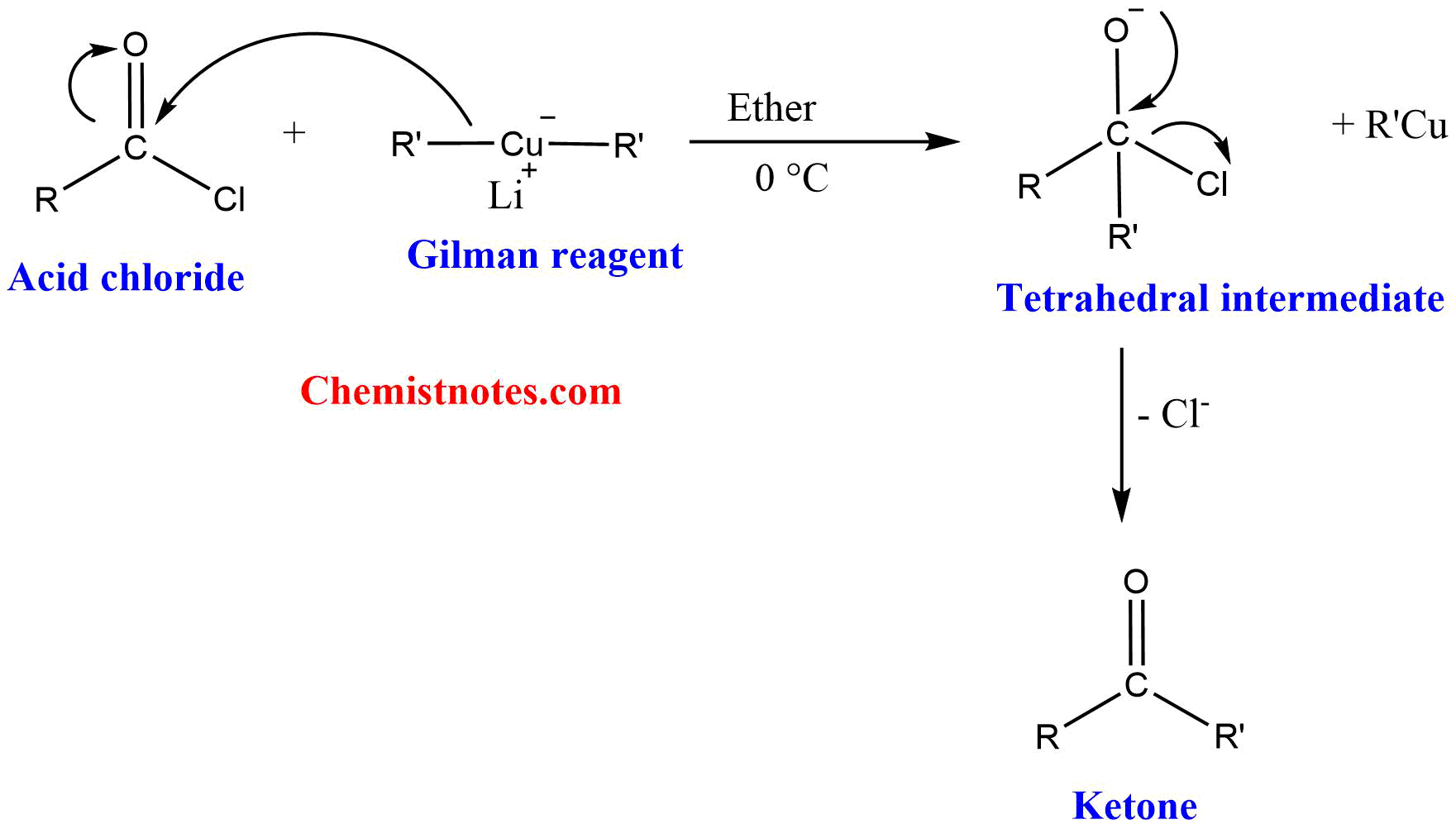
Mechanism of Gilman reagent with acid chlorides
Gilman reagent (lithium dialkylcuprate) acts as a nucleophile and attacks the carbonyl center to form a tetrahedral intermediate. The tetrahedral intermediate loses chloride ion to form a ketone.
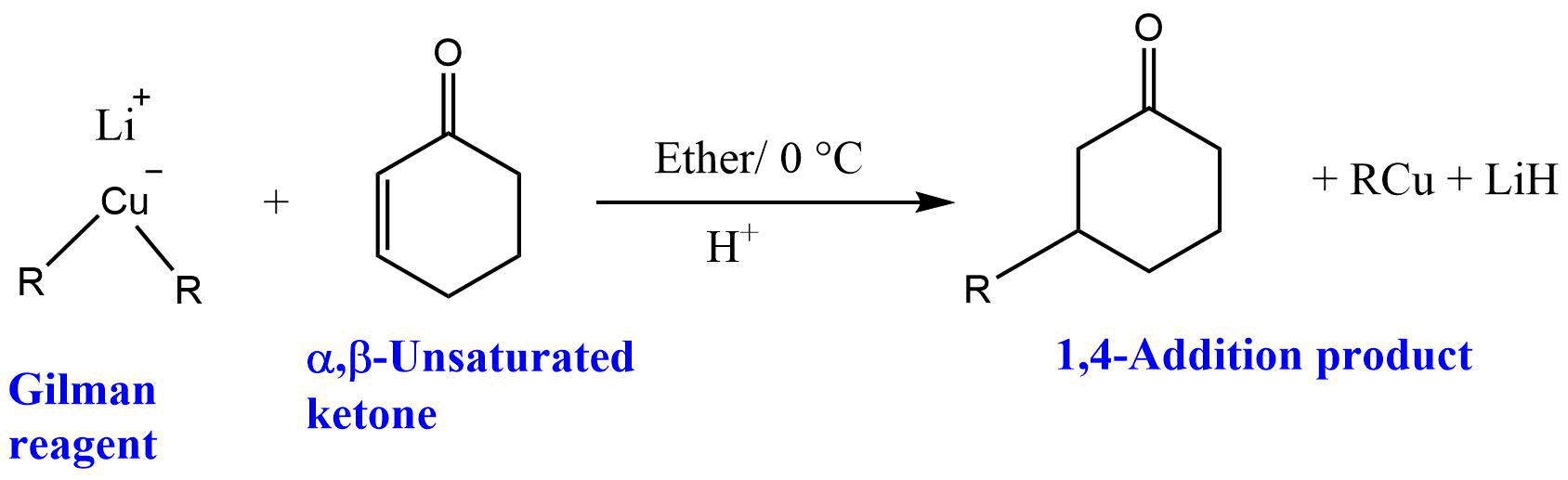
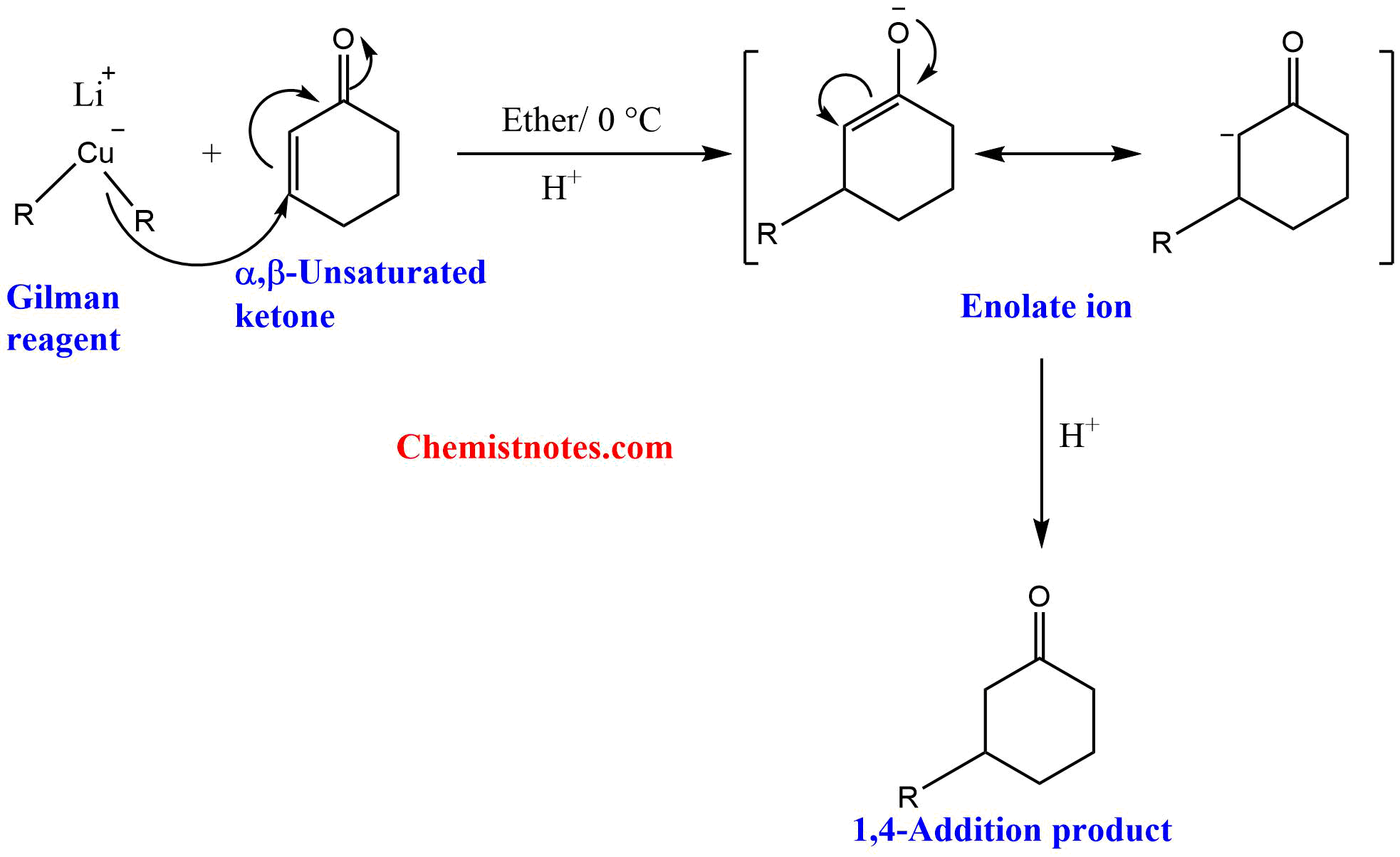
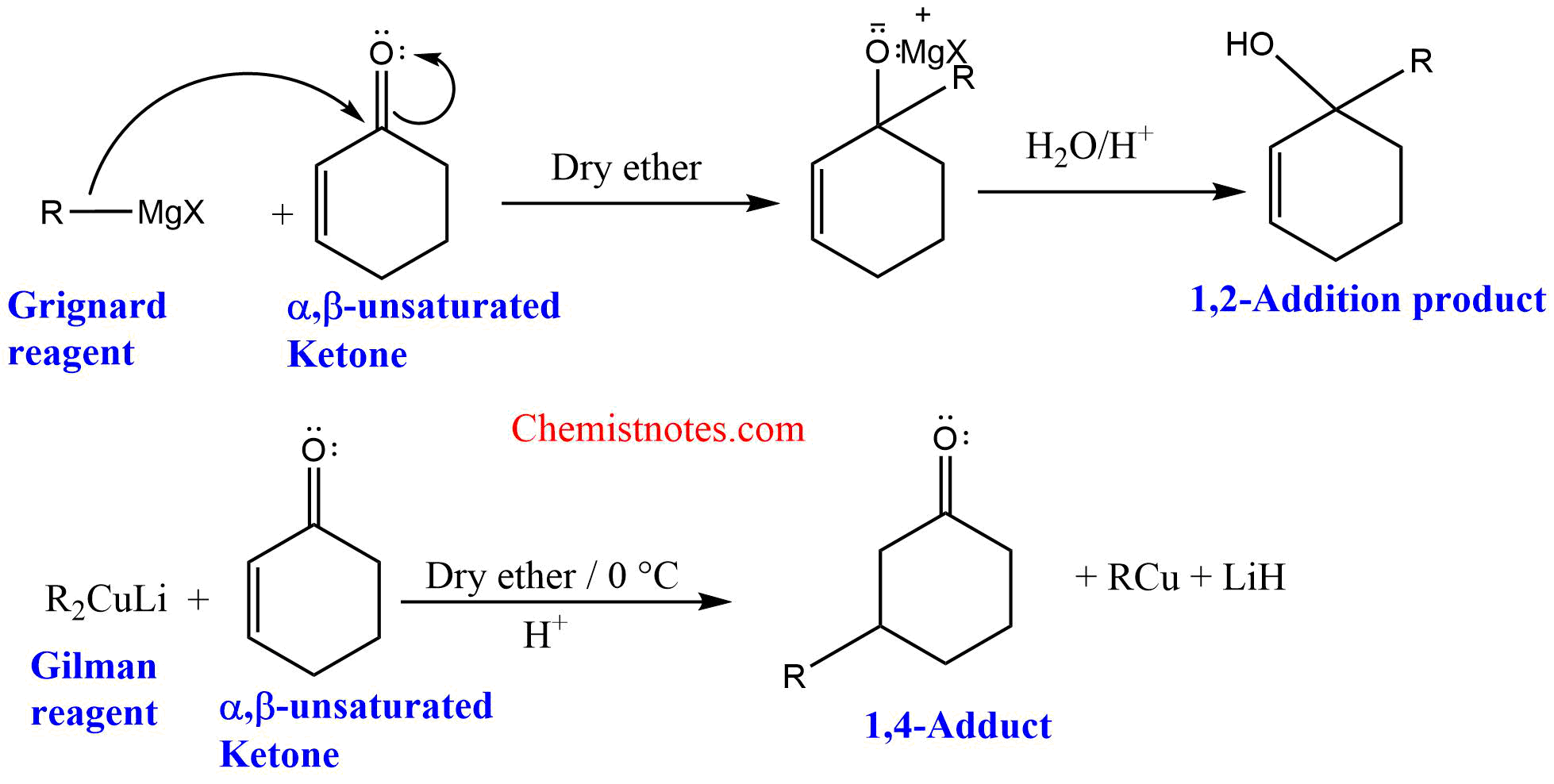
Reaction of Gilman reagent with α,β-unsaturated ketones
Gilman reagent reacts with α,β-unsaturated ketones to give 1,4-addition products.
Mechanism of Gilman reagent with α,β-unsaturated ketones
Initially, the nucleophile from the Gilman reagent attacks the carbon-carbon double bond of the α,β-unsaturated ketones, forming resonance-stabilizeded enolate ion. The enolate ion on acidification gives a 1,4-addition product.
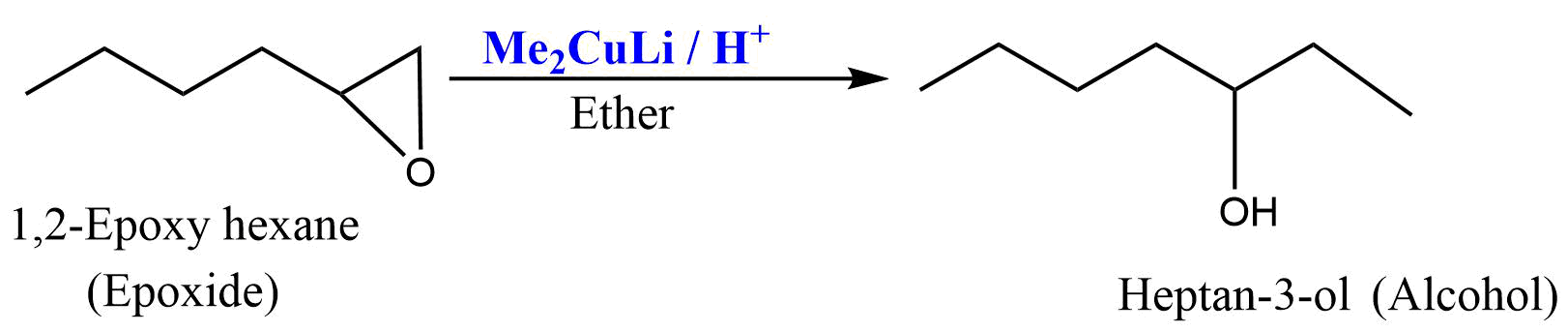
Reaction with epoxide
Gilman reagent attacks at the least substituted carbon atom of epoxide to give corresponding alcohol by SN2 type mechanism.
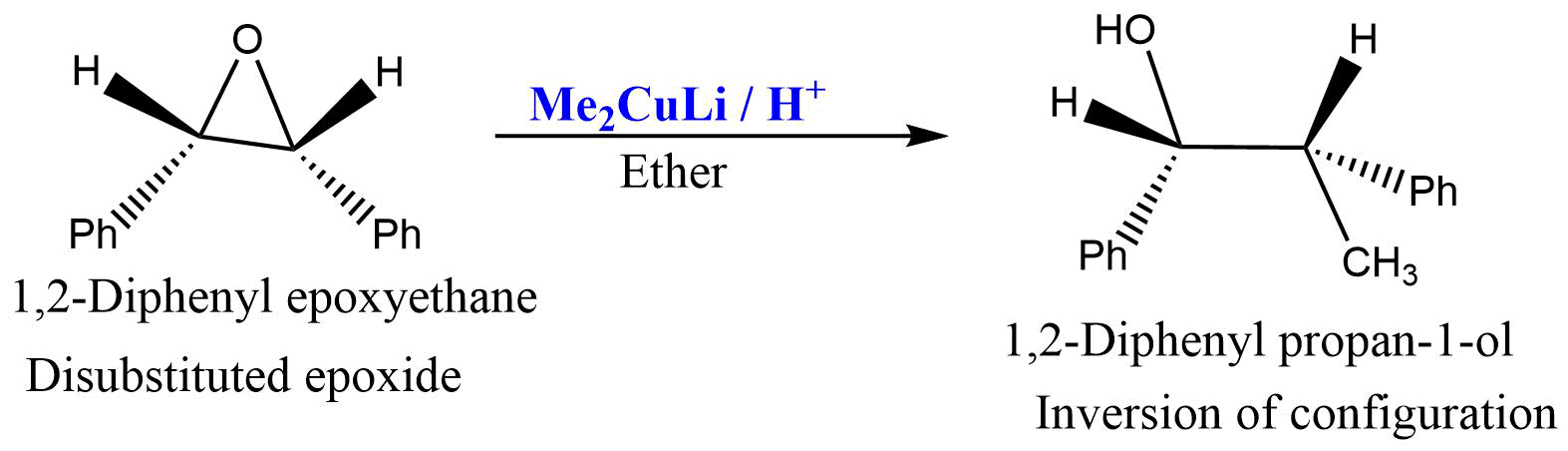
Since the reaction proceeds through the SN2 type mechanism, in disubstituted epoxide inversion of configuration is observed at ring carbon.
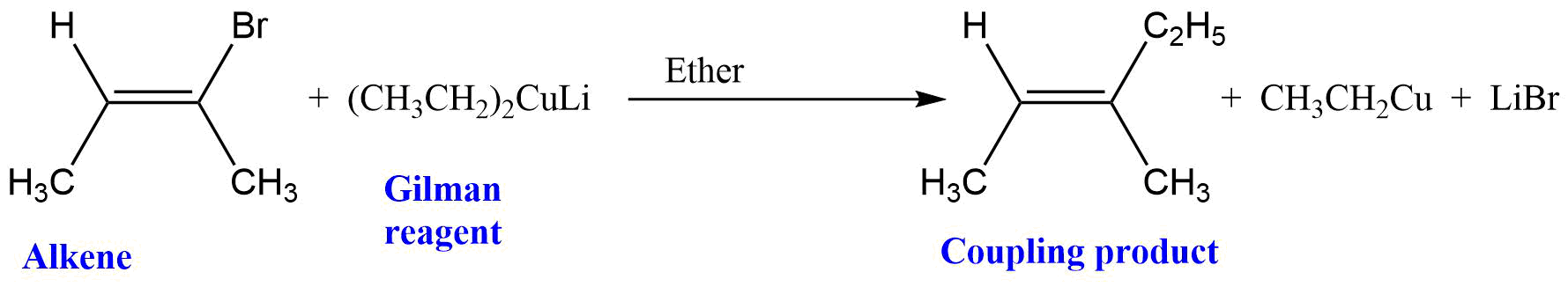
Reaction with vinyl halide
The Alkyl group from the Gilman reagent replaces the halogen attached to the alkene and aromatic ring to give a coupling product. This reaction is generally impossible with SN1 and SN2 reactions but the mechanism of the reaction is still unknown and is supposed to occur through free radical reaction.
Gilman reagent versus Grignard reagent
Since the C-Cu bond is less polarized than the C-Mg bond, the Gilman reagent is a strong nucleophile and less reactive compared to the Grignard reagent.
1. The Grignard reagent gives addition product with ketones and other carbonyl compounds but the Gilman reagent is a weak nucleophile compared to the Grignard reagent and does not react with ketones.
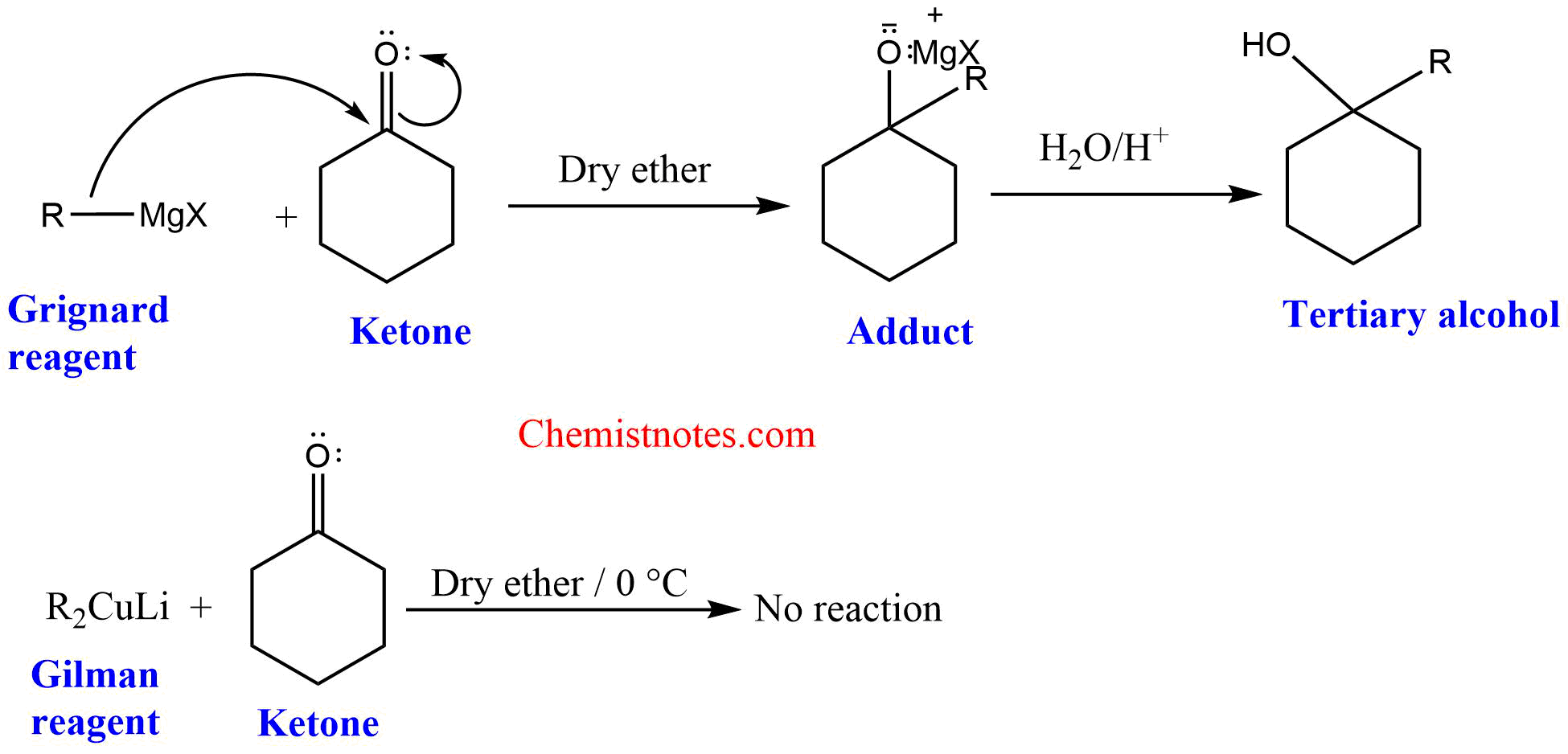
2. Grignard reagent gives tertiary alcohol with acyl chloride but Gilman reagent gives ketones with acyl chlorides.
3. Grignard reagent gives 1,2-addition products with α,β-unsaturated ketones but Gilman reagent gives 1,4-addition products.
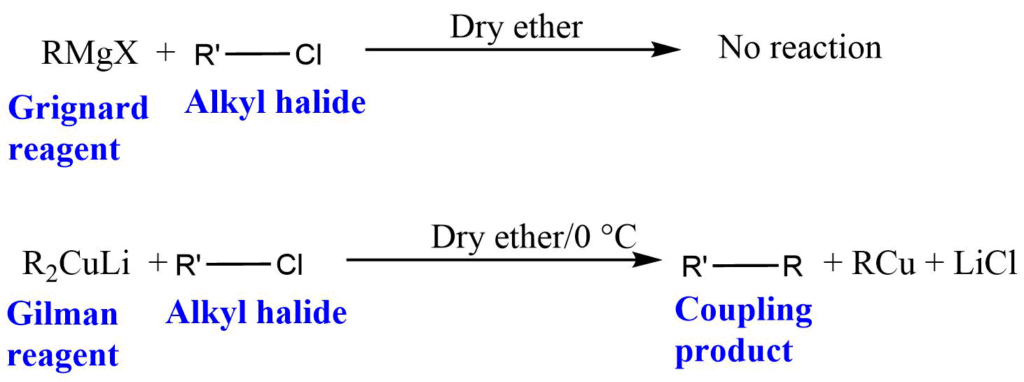
Which one is more nucleophilic Gilman reagent or Grignard reagent?
Gilman reagent is a strong nucleophile compared to Grignard reagent and gives coupling product with alkyl halides, whereas Grignard reagent does not react with alkyl halides.
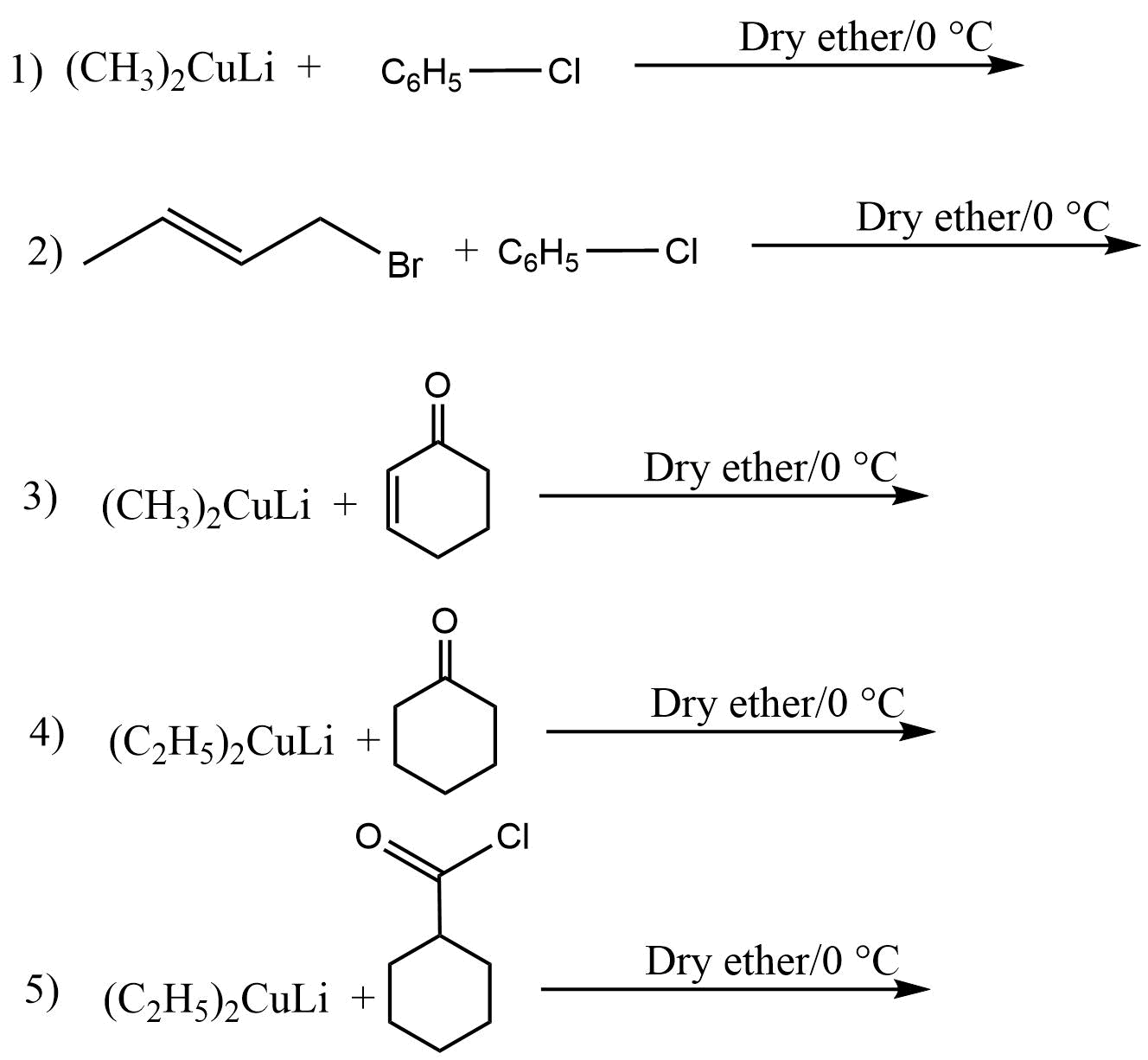
Gilman reagent practice problem
Complete the following reactions.
FAQs/MCQs:
Gilman reagent form carbon-carbon bonds through coupling with
Organochlorides, bromides, and iodides.
Published by: Siddha Raj Upadhyaya(Gurudev)
Gilman reagent video
References
- The Preparation of Methylcopper and some Observations on the Decomposition of Organocopper Compounds
Henry Gilman, Reuben G. Jones, and L. A. Woods
The Journal of Organic Chemistry 1952 17 (12), 1630-1634
DOI:1021/jo50012a009 - Chemistry of carbanions. XVII. Addition of methyl organometallic reagents to cyclohexenone derivatives
Herbert O. House and William F. Fischer Jr.
The Journal of Organic Chemistry 1968 33 (3), 949-956
DOI: 1021/jo01267a004 - Conjugate Addition Reactions of Organocopper Reagents
Gary H. Posner
React. 1972, 19, 1-114
DOI: 10.1002/0471264180.or019.01 - Carbon-carbon bond formation by selective coupling of n-alkylcopper reagents with organic halides
Elias J. Corey and Gary H. Posner
Journal of the American Chemical Society 1968, 90 (20), 5615-5616
DOI: 1021/ja01022a058 - Reaction of lithium dialkyl- and diarylcuprates with organic halides
George M. Whitesides, William F. Fischer Jr., Joseph San Filippo Jr., Robert W. Bashe, and Herbert O. House
Journal of the American Chemical Society 1969, 91 (17), 4871-4882
DOI: 1021/ja01045a049






