Table of Contents
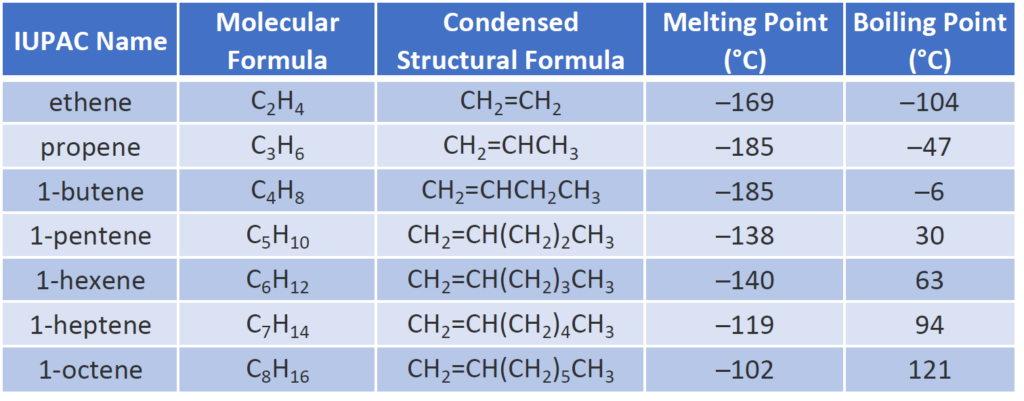
ToggleTest of Alkenes are the test of hydrocarbon which are unsaturated having double between carbon and hydrogen and also known as olefins. The stem, which shows how many carbon atoms an alkene has before the suffix -ene is added, is used to name both straight-chain and branched alkenes. The prefix is preceded by a location number that designates the carbon number on which the double bond begins. The terms “1-butene” and “2-butene” denote a four-carbon chain with a double bond between carbons 1 and 2, respectively, and a four-carbon chain with a double bond between carbons 2 and 3.
What are Test of Alkene?
Alkene is a hydrocarbon having a carbon–carbon double bond in organic chemistry. It is possible for the double bond to be internal or terminal. α-olefins are another name for terminal alkenes.
The name “olefin” should be used for the general class of cyclic or acyclic hydrocarbons with one or more double bonds, while “alkene” should only be used for acyclic hydrocarbons with one double bond. For acyclic hydrocarbons with two or more double bonds, use polyene or alkadiene, etc.; for cyclic hydrocarbons, use cycloalkene, cycloalkadiene, etc.
Properties of alkene
The properties of alkenes are:
- Alkenes are generally colorless, nonpolar.
- They are often called as olefins.
- They have sp2 hybridization.
- Have stronger smell than alkanes
- The melting and boiling point of alkene (ethene) are -169 and -104 respectively.
- When a C=C bond is stretched, it will give rise to an infrared absorption peak between 1670 and 1600 cm−1, and when it is bowed, it absorbs light between 1000 and 650 cm−1.
- Alkenes burn to produce carbon dioxide and water, just like the majority of other hydrocarbons.
How they are differ from alkane and alkyne?
Saturated hydrocarbons, or hydrocarbons with only one bond, are what the alkanes are. One or more carbon-carbon double bonds can be found in alkenes. Alkynes have one or more triple bonds between carbon atoms. Alkynes are hydrocarbons with one or more triple bonds, whereas alkenes are hydrocarbons with one or more double bonds. These chemicals follow naming guidelines that are comparable to those for alkanes.
Test for alkene
There are various test for alkenes. Let us discuss one by one:
- Reaction with Bromine water
- Reaction with acidified potassium permanganate
- Combustion
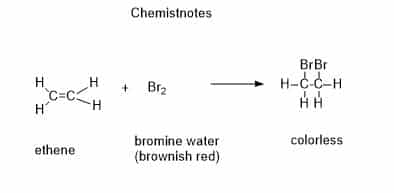
1. Reaction with Bromine water
Unsaturated hydrocarbons with one or more carbon-carbon double bonds are known as alkenes. Additionally, alkynes with a triple bond between carbon atoms may come across. Reactivity of unsaturated molecules is significantly higher than that of equivalent saturated alkanes. The first two assays for identifying unsaturated chemicals operate on this concept. Shaking the chemical with some bromine water is the most popular test. The initial orange solution rapidly loses its color when an alkene is added.
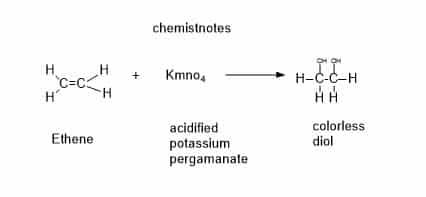
2. Reaction with acidified KMnO4
Shaking the chemical in a potassium manganate (VII) solution that has been acidified with diluted sulfuric acid is a similar test. Alkenes cause the initially purple solution to become discolored. The potassium manganate (VII) solution must be thoroughly acidified to avoid the possibility of a brown coloration. It will be effective to combine 0.02M potassium manganate (VII) and 1M sulfuric acid 50:50.
Combustion
Burning the material yields additional proof that it is unsaturated. Unsaturated chemicals typically burn bright and sooty. Given that unsaturation reduces the quantity of hydrogen atoms in a chemical, this may not come as a surprise. This raises the carbon content, and the sooty, bright flame is produced by the carbon. The more unsaturation there is, the more important this effect becomes.
Reference
http://Wade, L.G. (2006). Organic Chemistry (6th ed.). Pearson Prentice Hall






