Table of Contents
ToggleThe octant rule is one of the first and arguably the most effective of the several chirality sector rules that connect the cotton effect to organic stereochemistry. It establishes an absolute configuration or stereochemistry from the sign and intensity of the cotton effect and is unquestionably the most well-known and often-utilized rule.
Define octant rule
An octant rule is an empirical generation that links the arrangement of the chiral centers near the carbonyl chromophore to the sign of the Cotton effect of the chromophore as observed at about 300 nm in saturated cyclic ketones.
The study of the properties of chiral ketones began around 1954 at Wayne State University in the laboratory of Djerassi, which coincided with the understanding of conformational effects. Djerassi, Moscowitz, Woodward, Moffitt, and Klyne developed the formulation based on extensive optical rotatory dispersion data for n-π*. The octant rule mainly governs the cotton effect, which is observed in the case of chiral ketones.
Octant rule for ketones
Many studies have been carried out on steroidal ketones. The position of carbonyl groups with different substituents in the steroidal backbones shows the cotton effect, which helps in the determination of the conformation or configuration of numerous substituent groups. The main reason for choosing the steroid backbone was to minimize the conformational ambiguities and to allow the easy determination of the configuration and conformational effects of the different substituent groups. Besides, the carbonyl chromophore was chosen because of two factors:
- The excitation of the carbonyl chromophore due to n π* is in the readily accessible region having a UV absorption wavelength of 300 nm, and the next absorption band of higher energy (λmax 190 nm) is so far away that it removes the problem of overlapping and confusing the nature of the transition under study.
- The problems obtained experimentally during the measurement of the rotation from the absorption band are avoided due to the weakness of the n-π* transition.
In its most basic form, the rule states that a set of coordinates is drawn across a carbonyl group, with the origin in the middle of the carbonyl bond and the z-axis collinear with the bond. The xz and yz planes (two C2v symmetry planes), which are the nodal planes of the molecular orbital, split the carbonyl chromophore. The coordinate system divides the area surrounding the carbonyl group into eight regions (octants or sectors), four front sectors, and four sectors as shown in the figure below:
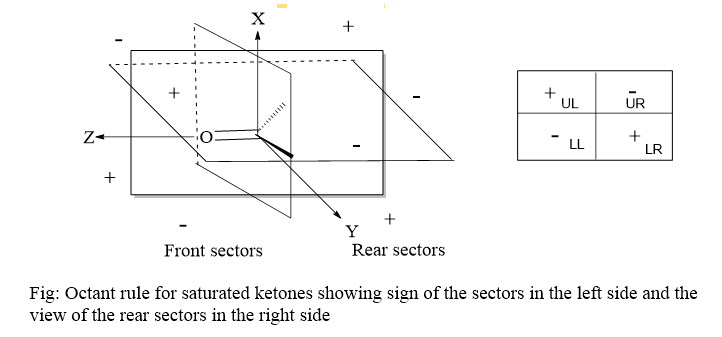
The main point of this rule is that the atom lying in the vicinity of the carbonyl group, at any point, let’s say P(x,y,z), bears a contribution and determines the sign of the cotton effect (n-π*) depending on its location. For example, the atom that is present in the lower right rear sector with coordinates -x, +y, -z, shows a positive cotton effect as it lies in the positive sector in the left-handed coordinate system.
Octant rule for cyclohexanone
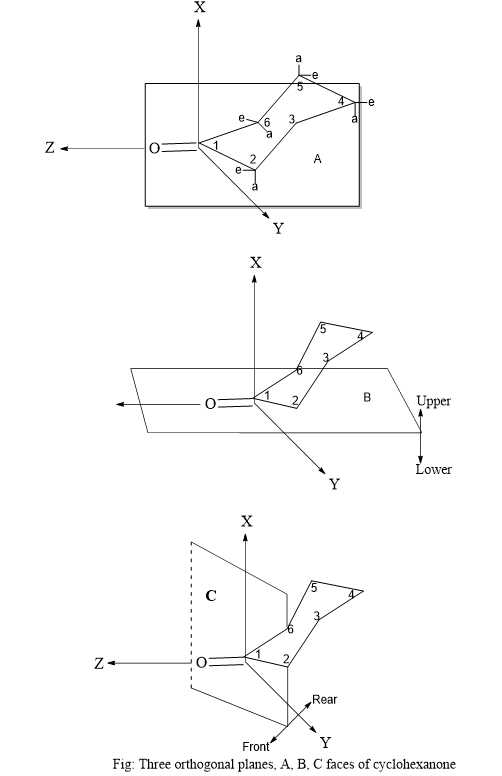
The Octant rule for cyclohexanone is one of the most studied types. The cyclohexanone molecule, with its well-known geometry and fixed conformations, was the first compound in which the octant rule was followed. The cyclohexanone molecule is oriented in the three-dimensional coordinate system, or three orthogonal planes A, B, and C. These planes basically split the carbonyl group into eight sectors in the same way as described above and are shown in the figure below:
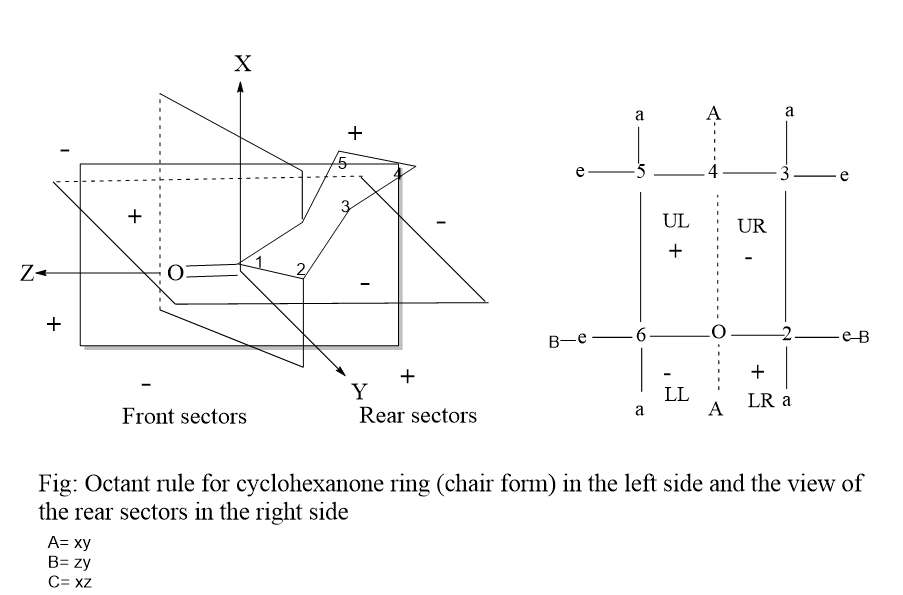
In the figure, the carbonyl group is the origin of the three-dimensional coordinate system and the Z-axis is collinear with the carbonyl group. Xy, zy planes, and xz are denoted as ‘A’, ‘B’, and ‘C’ planes respectively.
Plane A passes through the carbonyl group and carbon number 4 bisecting the cyclohexanone and the second horizontal Plane B passes through carbon numbers 2 and 6 and also contains the carbonyl group, they are denoted as lower right and lower left. Plane C passes through the carbon-oxygen bond at the right angle at the midpoint of the carbonyl group and separates the rear octants from the front octants and in most of the ketones, the substituents lie in rear octant regions behind the carbonyl group.
The two planes (A and B) split the space around the carbonyl group into four quadrants and the four rear quadrants are denoted as upper left (UL), upper right (UR), lower left (LL), and lower right (LR) as shown in the above figure and these sectors are denoted with (+) and (-) sign.
Note: Some ketones, like 1-keto and 11-keto steroids, contain some substituents in front octants. For the majority of the cases, the octant rule only needs to take the rear octants into account. Any unique circumstances that require the front octants to be considered will be clear from the ketone’s structure.
Octant rule in Stereochemistry
The octant rule demonstrates the different signs of the cotton effect depending on the substituents lying in different octants and is considered as:
- The substituents present near the nodal planes show no contributions to the cotton effect like in the case of cyclohexanone, the groups present at C(2), C(3), C(5), and C(6) can make such contributions. Furthermore, the equatorial substituents at C(2) and C(6) do not have any effect (or little) because they lie in the B plane (yz plane) and the substituents at C(4) also lie in the A plane and thus, have no effect.
- The substituents that lie at the rear lower right octant give a positive contribution to the cotton effect and the sign is also positive for the upper left octant at the rear side. Similarly, the substitution in the rear upper right and rear lower left makes a negative contribution
- The substituents present at the lower right and upper left axially give a positive cotton effect, and those present at the lower left and upper right axially show a negative contribution to the cotton effect.
- It is occasionally feasible to provide a semiquantitative evaluation of contributions that occur in many rings because they are additive.
- As previously expected, contributions from carbon atoms, Sulphur, and halogen atoms are the same but because of their substantially greater atomic refraction, the halogens (except fluorine) dominate the cotton effect, totally outweighing contributions from the alkyl groups. It is noteworthy that out of all the halogens, fluorine has the lowest atomic refraction; in fact, it is less than that of hydrogen.
- Groups that include both oxygen and nitrogen may behave in an octant or antioctant (inverse) manner. For instance, the -N(CH3)3+ group demonstrates anti-octant activity.
(Cosignate (Octant) is the effect caused by a specific substituent if the sign occurred from the product of the coordinates coincides with its contribution in circular dichroism intensity and Dissignate (antioctant) is the effect that shows a contrast in the relationship in the sign of the contribution to the circular dichroism and the sign from the cartesian coordinates).
Axial haloketone rule
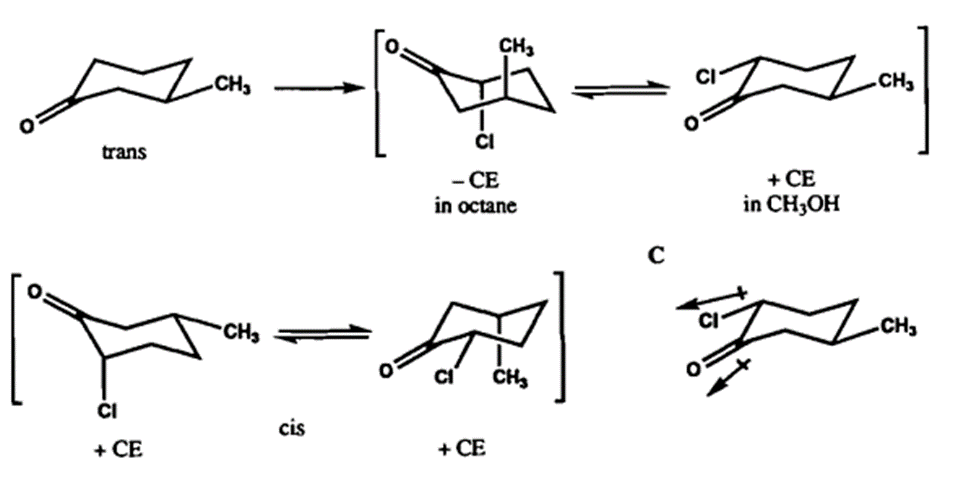
The axial haloketone rule is the special case of the octant rule and it determines the sign of the cotton effect based on the conformation of α-halogens in α-halocycloketones. The equatorial α-halogens do not show much effect in the cotton effect but the axial α-halogens change the sign of the cotton effect according to the axial haloketone rule.
When the carbonyl carbon is positioned at the head of a chair or boat form of cyclohexanone, the molecule is seen along the axis of the carbonyl bond for the purpose of this rule. The cotton effect is expected to be positive if the halogen is on to the observer’s right, and negative if it is to their left.
Basically, the sign of the cotton effect depends on the conformation, configuration, and constitution of the haloketone in the area of the carbonyl group.
The negative cotton effect is shown mostly by trans stereochemistry in non-polar solvent but in polar solvent, the positive effect is shown. The main reason behind this could be that the shifting of conformation has taken place in the case of more polar solvents due to low repulsion between the equatorial carbonyl and chlorine atoms. This rule can be illustrated as:
Application of Octant Rules with examples
The application of the octant rule with some examples is listed below:
- Determination of absolute configuration of ketones:
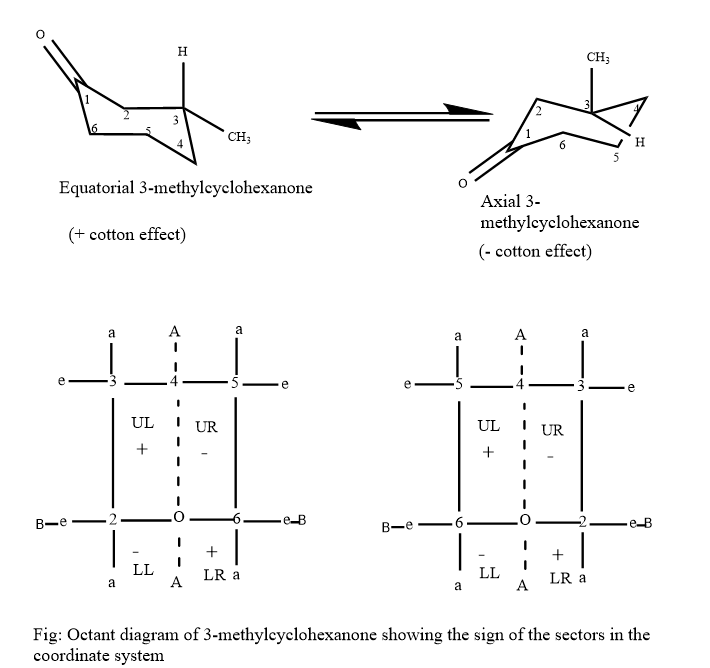
Octant diagram plays a crucial role in the determination of the configuration of ketones which can be easily justified with the example of 3-methylcyclohexanone, which is a chiral and optically active compound.
Since equatorial 3-methylcyclohexanone is more stable predominating the equatorial conformation and its optical rotation can be determined from a polarimeter which results in a laevorotatory compound. So, in this compound based on the octant rule, the methyl group lies in the upper left octant in equatorial 3-methylcyclohexanone which gives a positive effect.
Similarly, the methyl group in the axial 3-methylcyclohexanone lies in the upper right octant demonstrating a negative effect but overall, 3-methylcyclohexanone demonstrates a positive effect, which means the methyl group is in the equatorial position.
- Determination of optical properties of chromophores
A chiral chromophore that exhibits circular dichroism at ultra-violet absorption bands is optically active and shows the cotton effect. An intrinsically achiral chromophore requires extrachromophoric disruption to display circular dichroism: in contrast, an intrinsically chiral chromophore’s circular dichroism originates from the chromophore’s dissymmetry. Generally, the former’s cotton effect has a magnitude that is one or two orders of magnitude more than the latter.
For example, the carbonyl chromophore can be a very sensitive chiroptical probe since its optical activity is from chiral perturbers located inside the molecule. According to the Octant rule, this means that it provides a window for the detection of extrachromophoric stereochemistry. - Conformational and stereochemistry analysis of saturated ketones
Some examples of conformational and stereochemistry analysis of saturated ketones are shown in the table below:
Table: Chair conformers of 2-oxo-p-menthanol along with octant diagram and sign of cotton effect (Retrieved from https://doi.org/10.1016/S0167-9244(08)70178-1)
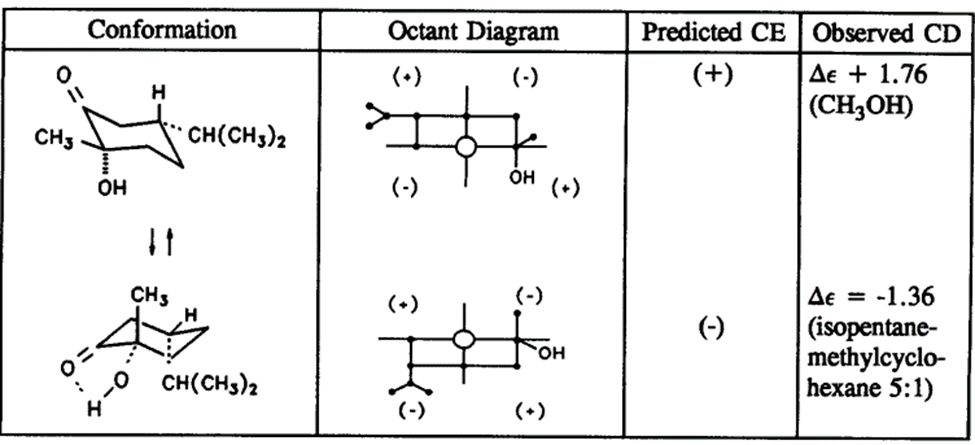
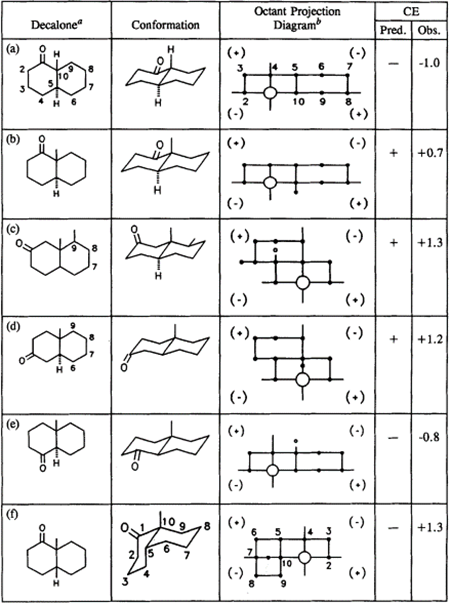
Table: Conformation of Decalones with octant diagrams and predicted cotton effects.(Retrieved from https://doi.org/10.1016/S0167-9244(08)70178-1 )
- Determination of absolute stereochemistry of αβ-unsaturated ketones.
- Synthesis of the (1s,5s)-dimethyladamantan-2-one form the optically active compound, adamantane shows no contribution in cotton effect by octant and quadrant rules.
References
- Lightner, David A., and Vien Van Toan. “The Octant Rule. XX.” Tetrahedron 43, no. 21 (1987): 4905–16. https://doi.org/10.1016/S0040-4020(01)87672-9.
- Lightner, David A., Thomas D. Bouman, W. M. Donald. Wijekoon, and Aage E. Hansen. “The Octant Rule. 18. Mechanism of Ketone n.Fwdarw. .Pi.* Optical Activity. Experimental and Computed Chiroptical Properties of 4-Axial and 4-Equatorial Alkyladamantan-2-Ones.” Journal of the American Chemical Society 108, no. 15 (July 1986): 4484–97. https://doi.org/10.1021/ja00275a039.
- Lightner, D.A. “Chapter 5 Determination of Absolute Configuration by CD. Applications of the Octant Rule and the Exciton Chirality Rule.” In Techniques and Instrumentation in Analytical Chemistry, 14:131–74. Elsevier, 1994. https://doi.org/10.1016/S0167-9244(08)70178-1.
- McKelvey, Ronald D. “Stereochemistry of Organic Compounds ( Eliel, Ernest L.; Wilen, Samuel H.).” Journal of Chemical Education 73, no. 8 (August 1996): A174. https://doi.org/10.1021/ed073pA174.2.
- Mandal, Dipak K. “Cyclic Molecules: Configuration and Conformation.” In Stereochemistry and Organic Reactions, 125–212. Elsevier, 2021. https://doi.org/10.1016/B978-0-12-824092-2.00003-4.






