Table of Contents
ToggleAromaticity rules is the property of the planar ring compound in which p-orbital allow the cyclic delocalization of pi-electron. They exhibit certain characteristics properties that are quite different from those of aliphatic and acyclic compounds. Aromatic rings are associated with high thermodynamics stability. They do not give addition reactions but they undergo readily certain electrophilic substitution reactions.
Huckle’s Rule of Aromaticity (4n+2 rule)
German physicist Erich Huckel formulated a simple rule on the basis of molecular orbital theory, the presence of (4n+2) delocalized pi electrons in a planar cyclic system is the cause of aromaticity and is known as Huckel’s rule. Here, provided ‘n’ is a whole number of integer and maybe 0,1,2,………etc.
Thus the presence of delocalized pi electrons is not enough for a molecule to show aromaticity. In fact, there must be a particular number of pi electrons i.e. 2 or 6 or 10, etc. This requirement is called (4n+2) rule.
According to this rule, the following criteria are necessary:
- The compounds must have cyclic planar structure
- The compounds must contain cyclic cloud of delocalised pi-electrons above and below the plane.
- All ring carbon atoms of compound must be sp2 hybridized.
- The compound must contain huckel number 2,6,10,14 or 18 pi electrons.
Aromaticity Examples
Aromaticity of Benzene
Benzene is the parent aromatic compound. Therefore, benzene and compounds that resemble benzene in chemical behavior are known as aromatic compounds. The benzene molecule has six carbon atoms arranged in a closed chain and most of the aromatic compounds are derived from it.
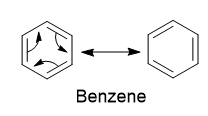
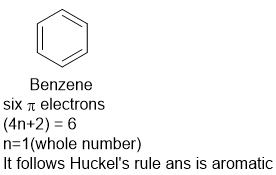
Benzene and its derivatives having 6 pi electrons are aromatic as they obey Huckel’s rule.
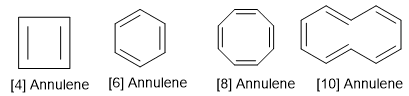
Annulene Aromaticity
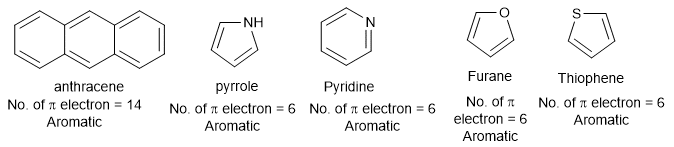
Aromaticity of Anthracene/pyrrole/pyridine/furane/Thiophene
FAQs
How to determine aromaticity?
Aromaticity is determined by following Huckle’s (4n+2) rule.
What is aromaticity of benzene?
Benzene and its derivatives having 6 pi electrons are aromatic as they obey Huckel’s rule.






