Table of Contents
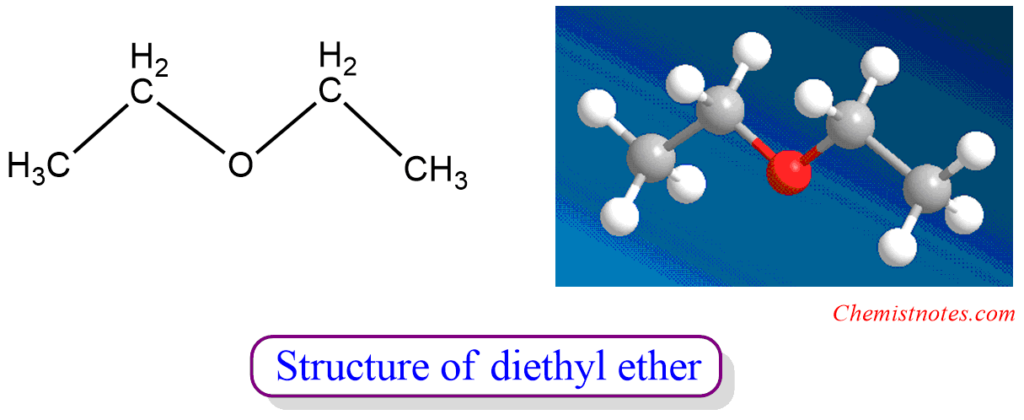
ToggleDiethyl ether is an ether that has an oxygen atom bonded to two ethyl groups. It is a non-polar solvent which is the most important representative of the ether class.
Preparation of Diethyl ether
It can be prepared by any of the general methods of preparation of ethers.
Laboratory Preparation
It is prepared in the laboratory and on a large scale by heating excess of ethyl alcohol with concentrated sulphuric acid to 140oC. The involved reaction can be represented as;
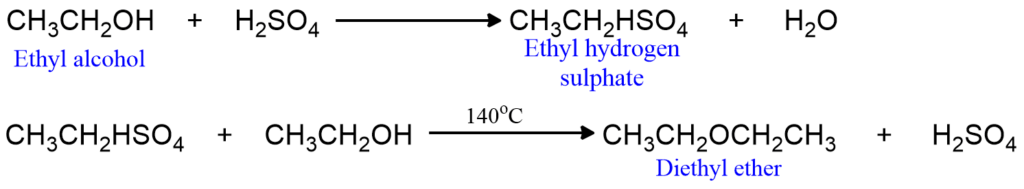
About 50 ml Conc. H2SO4 is mixed slowly with 50 ml of absolute alcohol taken in a 250 ml distillation flask with shaking and cooling. The flask is then fitted with a dropping funnel, a thermometer, and a water condenser which in turn is connected to a receiver immersed in ice-cold water. The whole apparatus is made air-tight.
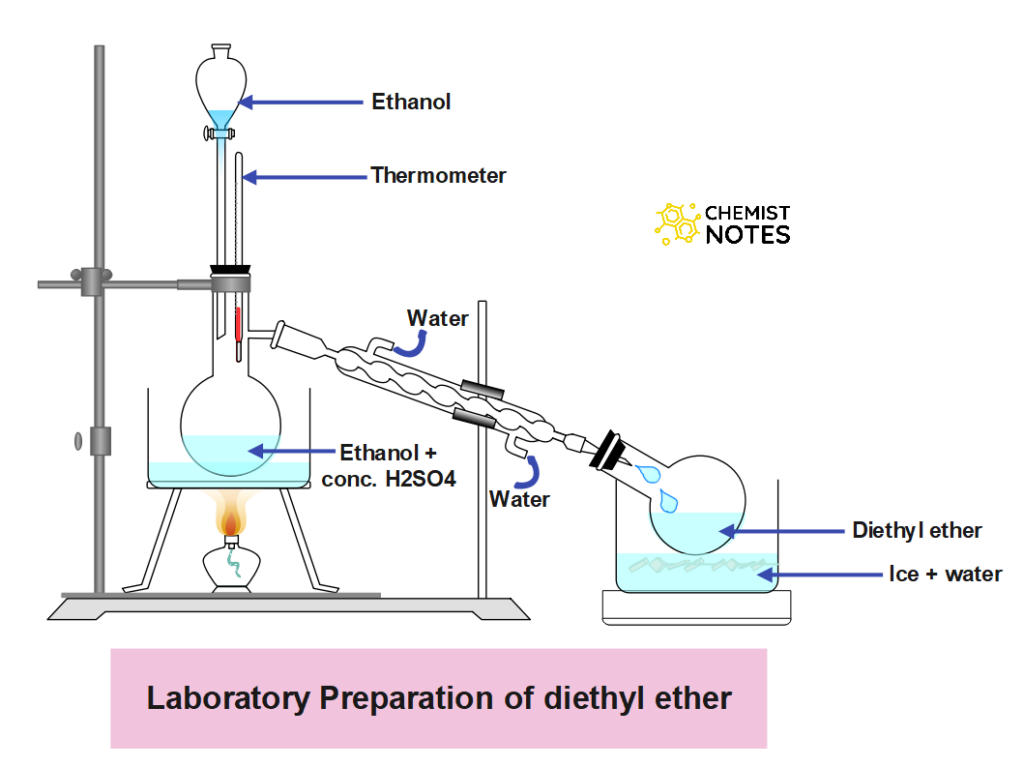
Now the flask is heated on a sand bath. When the temperature of the content in the flask reaches 140oC, ether begins to distill over. Alcohol is now run in form dropping at the same rate at which ether distills over.
Ether so obtained is impure and is contaminated with ethyl alcohol, water, and sulphuric acid. It is purified by first shaking with dilute NaOH which removes sulpurous acid. The ethereal layer is then separated again and allowed to stand over anhydrous calcium chloride for drying and distilled over sodium to get pure ether.
Properties of Diethyl ether
Physical properties
- Diethyl ether is a colourless highly volatile liquid having a pleasant odor and sweetish burning taste.
- It has a boiling point of 34.6oC.
- At 25oC, it is soluble in water to the extent of 8%.
- On inhalation, its vapors produce general anesthesia.
Chemical properties
It gives all the general reactions of ethers.
Uses of Diethyl ether
- It is extensively used as a solvent both in laboratory and industry.
- It is an excellent inert medium for many organic reactions such as the Wurtz reaction, Grignard reaction, etc.
- It is used as a solvent for extracting organic compounds from their aqueous solution.
- Diethyl ether is one of the best-known anesthetics. It is superior to chloroform in that it produces unconsciousness without interfering much with the function of the heart and lungs.
- It is also used for producing powder alcohol.
- It is used as a refrigerant. A mixture of ether and dry ice gives a temperature as low as -77oC.
FAQS
Is diethyl ether polar?
It is a non-polar solvent which is the most important representative of the ether class.
Is diethyl ether soluble in water?
It is slightly soluble in water, with 6.05 g/100 ml of water at 25oc.
Is diethyl ether flammable?
It is a flammable liquid.
What is diethyl ether?
Diethyl ether is an ether that has an oxygen atom bonded to two ethyl groups.






