Table of Contents
ToggleWhen an alkyl halide solution in dry ethyl ether (C6H5)2O, is allowed to stand over turning of metallic magnesium, a rapid reaction occurs. The solution has become cloudy, begins to boil, and the magnesium metal gradually dissolves. The resulting solution is known as a Grignard reagent, after Victor Grignard who received the Nobel Prize in 1912 for its discovery.
It is one of the most diverse and helpful reagents known to organic chemists.
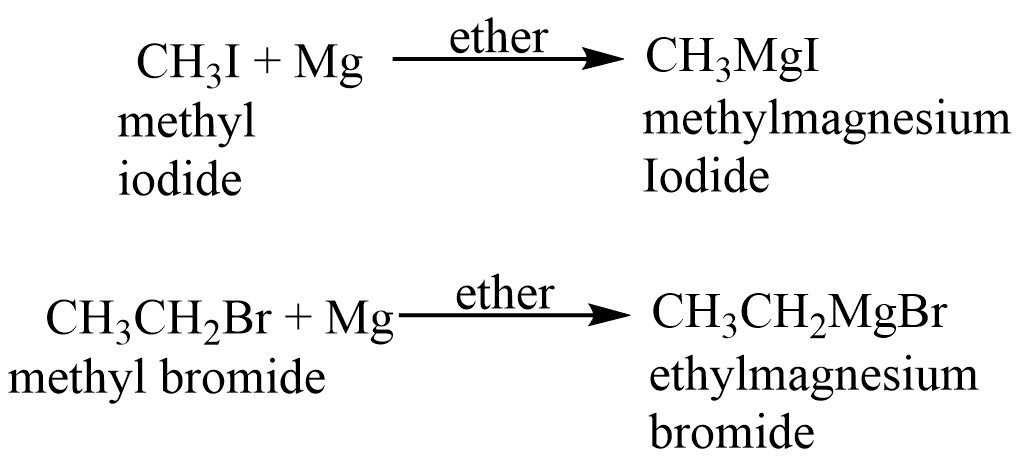
The Grignard reagent is also termed as alkyl magnesium halide and has the general formula RMgX. The covalent carbon-magnesium link is strongly polar, with carbon pulling electrons from electropositive magnesium; the magnesium-halogen bond is essentially ionic.
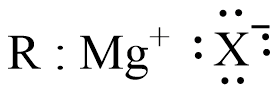
An Example of Grignard reagent
The alkyl group is intact throughout the synthesis of the reagent because magnesium is bonded to the same carbon that previously held halogen. Isopropyl chloride produces isopropyl magnesium chloride, whereas n-propyl chloride produces n-propyl magnesium chloride.
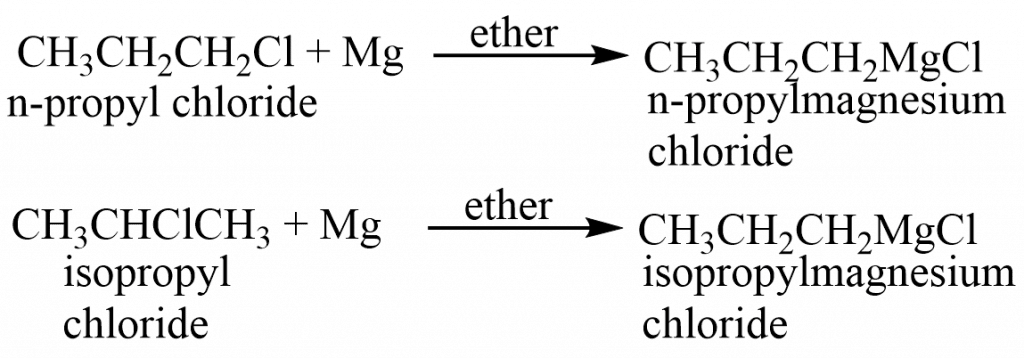
Grignard reaction
The reactivity of the Grignard reagent is quite high. It interacts with a wide range of inorganic chemicals, including water, carbon dioxide, and oxygen, as well as most organic molecules; in many situations, the reaction is the most efficient way to create a certain class of organic compounds.
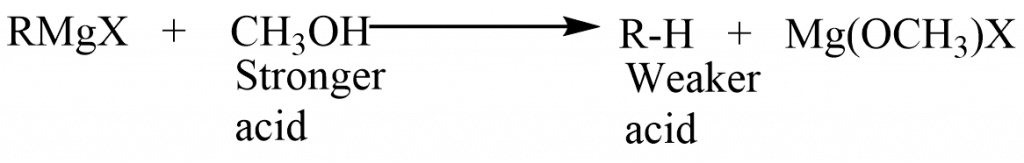
The Grignard reagent, like many other highly reactive organometallic compounds, reacts with water to generate an alkane. We can regard the Grignard reagent to be the magnesium salt, RMgX, of the extremely weak acid, R-H, because of the carbanion nature of the alkyl group.

The reaction is basically the displacement of R-H from its salt by the stronger acid, HOH. Because an alkane is such a weak acid, it gets displaced from the Grignard reagent by compounds that we would normally regard to be very weak acids or possibly not acids at all. Any compound containing hydrogen attached to oxygen or nitrogen is tremendously more acidic than an alkane and therefore can decompose the Grignard reagent.
Refrences:






