Table of Contents
TogglePhase transfer catalyst is one kind of catalyst that facilitates the transfer of one reacting species from one phase (aqueous phase) to another phase (organic phase) in order to carry out a reaction. Many chemical reactants can’t react or can’t collide due to the separation of these species by an interface. Such problems have been solved by using a type of catalyst known as phase transfer catalyst, also called PTC.
what is phase transfer catalyst?
When a chemical reaction is planned to carry out, sometimes one difficult situation may arise due to the immiscibility of reactants. In other words, reactants don’t mix. The reactants have to be in the same phase either in the aqueous or the organic phase. The reactant molecules must collide with each other for the reaction to occur.
If one of the reactants is water-soluble and another reactant is insoluble in water but soluble in the organic phase, on mixing these reactants the reaction may not take place because the reactant can not collide with each other when present in different phases. Then, how can we solve this problem?
In order to overcome such difficulty, Chemists have developed one special type of substance which facilitates the mixing of these reactants in the same phase by transferring from one phase to another phase. A substance that has the ability to transfer one of the reactants from one phase (say aqueous) to another phase ( say organic) having another reactant, to carry out a reaction is called phase transfer catalyst and phenomenon is called phase transfer catalysis.
Such types of catalysts are a useful tool not only in organic chemistry but also in inorganic chemistry, photochemistry, electrochemistry, and especially polymer chemistry. Moreover, It has helped chemists the ability to conduct reactions between organic compounds and strong inorganic oxidants such as permanganate, dichromate, hypochlorite, and hydrogen peroxide.
Note: This method is not limited to anions only, and a few works have been done in transferring cations, radicals, and molecules. Similarly, a reverse type of phase transfer catalysis has been reported. In this reverse type, the anion is transported in the aqueous phase from the organic phase.
Phase transfer catalyst example
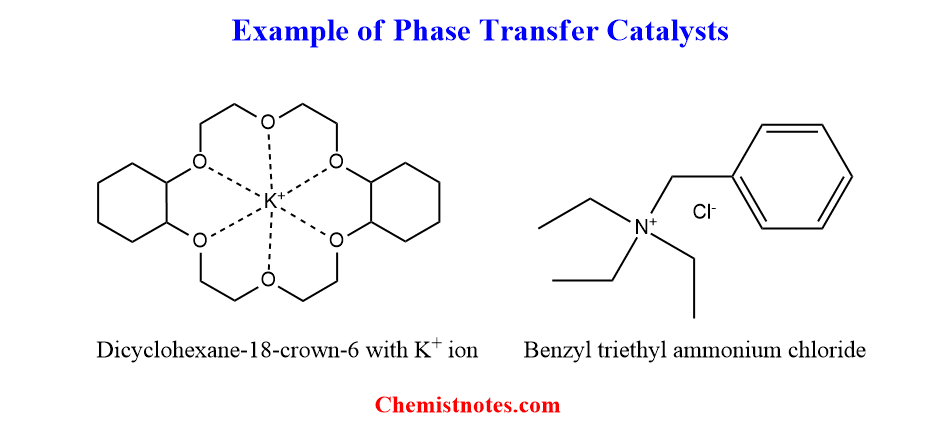
There are mainly two types of phase transfer catalysts.
- Quaternary ammonium or phosphonium Salts.
- Crown ethers and Cryptands.
These catalysts have somewhat slightly different actions but the effects are the same. Both types transfer the anions from one phase to another phase and allow them to be free to react with the substrate.
Some examples of phase transfer catalysts are shown below:
Phase transfer catalyst mechanism
When a nucleophilic substitution reaction is carried out between 1-chlorooctane and aqueous NaCN, the reaction doesn’t take place and no product is formed. Generally, the substrate for nucleophilic substitution is insoluble in water and other polar solvents, while nucleophile ( anions) is soluble in water but not in the substrate or other organic solvents. This is the reason why the reaction does not take place between the 1-chlorooctane and aqueous NaCN.
To carry out the above reaction, these reactants must be in the same phase and for this purpose, a special type of catalyst called phase transfer catalyst is used. When a small amount of an appropriate quaternary ammonium salt is added, the reaction proceeds and the product is formed. In this method, the catalyst carries the nucleophile (CN–) from the aqueous phase to the organic phase.
The mechanism of working of phase transfer catalyst is represented in the following figure, where Q+ is quaternary salt-containing sufficiently long alkyl groups or other organic structures to make soluble in the organic phase.
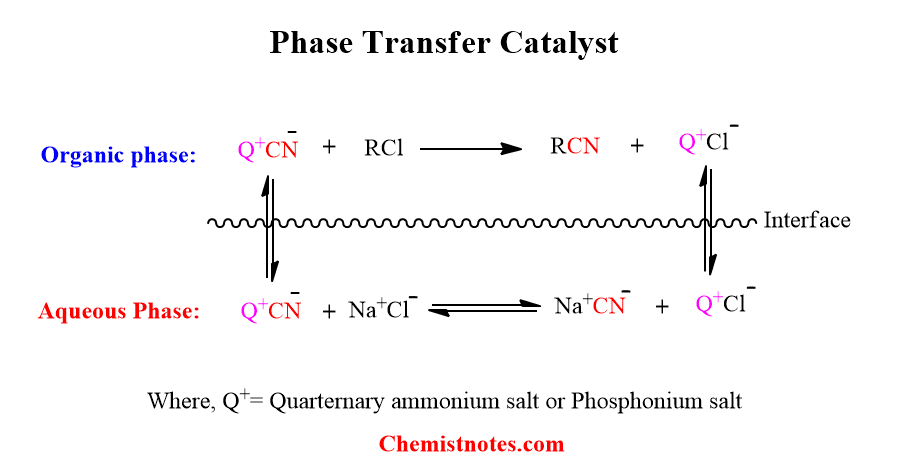
If a small amount of these salts are added, in the beginning, the CN– ions are carried into the organic phase from the aqueous phase by Q+ ion. In the organic phase, CN– ion reacts with 1-chlorooctane to produce 1-cyanooctane and Cl– ion. The Cl– ion then gets carried into the aqueous phase in the form of Q+Cl–, which undergoes exchange with CN– to regenerate Q+CN–.
If Q+ ions have such a low solubility in water, the exchange of ions takes place across the interface of the aqueous and organic phases.
Advantages of phase transfer catalyst
Some of the major advantages of such catalysts are listed below:
- It helps to reduce the consumption of organic solvents and raw materials.
- Using it, the reaction can be carried out in mild reaction conditions.
- It helps to control both reaction conditions, reaction rate, and yields.
Limitations of phase transfer catalyst
This catalyst may have some limitations, which are listed below:
- Ordinary tetraalkyl quarternary salts decompose at high temperatures i.e greater than 120-149 degrees Celcius.
- Even at low temperature under highly alkaline conditions, these catalyst decomposes.
- These salts undergo decomposition in the presence of highly nucleophilic anions such as phenoxide.
- Crown ethers and cryptands are stable under these conditions but are much more expensive than quaternary salts.
- For the transfer of divalent or trivalent anions, no superior catalysts have been introduced.
- One of the disadvantages of crown ether catalyst is that it is limited by hole size relationship. For example; 15-crown-5 has a hole size similar to sodium cation (Na+) diameter is selective for Na+ over other cations.
References:
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992.







2 Responses
It is helpful.
Thank you.