Table of Contents
ToggleWhat is a hydrocarbon?
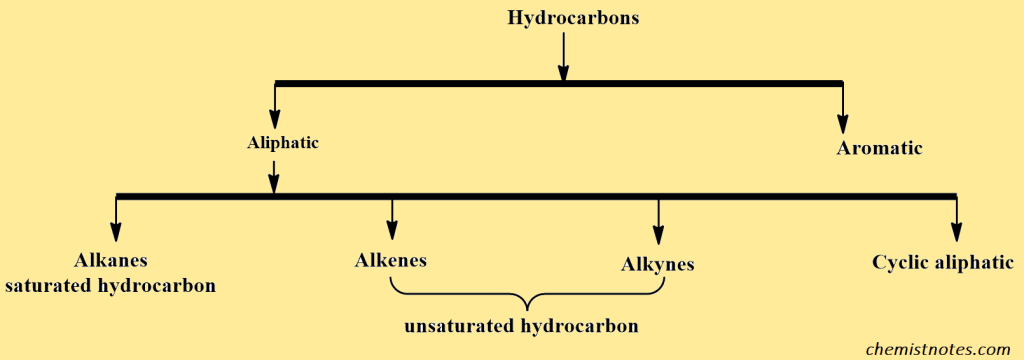
Hydrocarbons are organic compounds that only contain hydrogen and carbon. On the basis of structure, hydrocarbons are divided into two main classes: aliphatic and aromatic. Aliphatic hydrocarbons are further divided into alkanes, alkenes, and alkynes and their cyclic analogs (cycloalkanes, etc.)
Saturated hydrocarbon
The hydrocarbons that contain a carbon-carbon single bond are known as saturated hydrocarbons. For example, alkanes and cycloalkanes.
Unsaturated hydrocarbon
On the other hand, hydrocarbons that contain carbon-carbon double or triple bonds are known as unsaturated hydrocarbons. For example, alkenes, and alkynes.
Hydrocarbon formula
The General molecular formula for saturated hydrocarbon (alkanes) is CnH2n+2, the molecular formula for alkenes is CnH2n, the molecular formula for alkynes is CnH2n-2, the molecular formula for cyclic aliphatic hydrocarbons is CnH2n, and the molecular formula for aromatic hydrocarbons CnH2n-6.
| Class | General formula |
| Saturated hydrocarbon | CnH2n+2 |
| Alkenes | CnH2n |
| Alkynes | CnH2n-2 |
| Cyclic aliphatic | CnH2n |
| Aromatic | CnH2n-6 |
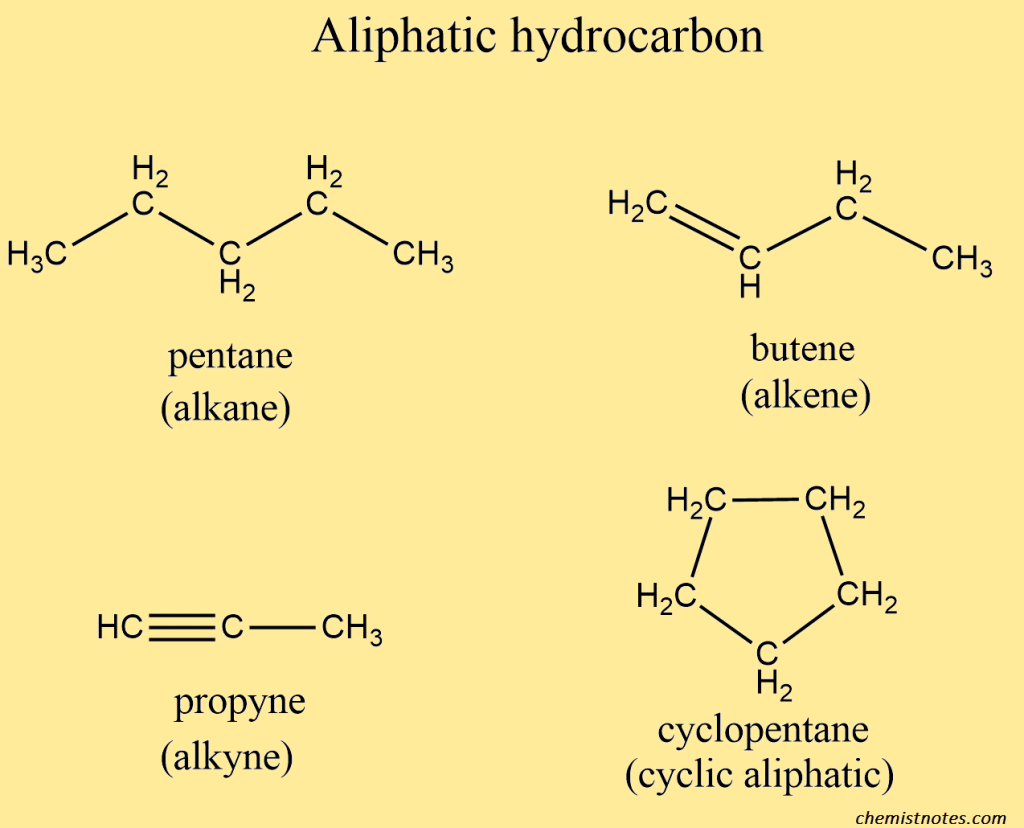
Aliphatic hydrocarbon
Aliphatic hydrocarbons are organic compounds made up of carbon and hydrogen atoms in straight chains, branched chains, or non-aromatic ring structures. Covalent bonds are used to connect carbon and hydrogen atoms. Aliphatic hydrocarbons are classified into two types based on the presence or absence of double bonds: saturated aliphatic hydrocarbons(alkanes) and unsaturated aliphatic hydrocarbons(alkenes, and alkynes).
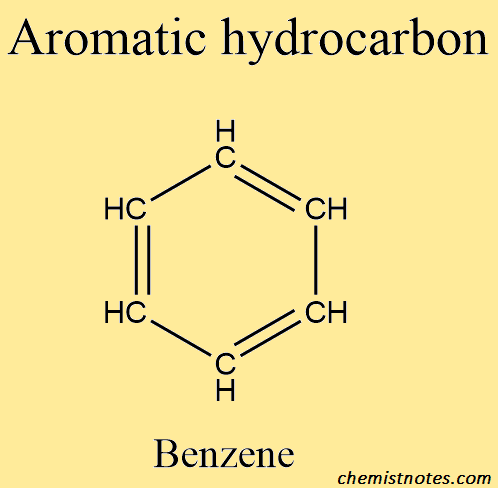
Aromatic hydrocarbon
Aromatic hydrocarbons, on the other hand, organize into a ring of carbon atoms. These are usually in the form of a single hexagon or multiples bonded together. Aromatic hydrocarbons are classified into two types: benzenoids and nonbenzenoids. If the aromatic hydrocarbon is a nonbenzenoid, it has either more than or less than six carbon atoms in the ring.
Sources of Hydrocarbon
Plants and animals were considered to be the only source of organic compounds until the beginning of the nineteenth century. However, the industrial revolution in Europe projected coal and petroleum as the major sources of organic compounds, particularly, hydrocarbons. Before we take up the isolation of hydrocarbons from petroleum or coal, let us study their origin and also their composition.
Coal contains aromatic hydrocarbons like benzene, toluene, xylene, naphthalene, and anthracene, along with some organic compounds containing sulfur and nitrogen. Petroleum is primarily a mixture of hydrocarbons, particularly alkanes, cycloalkanes, and some aromatic hydrocarbons.
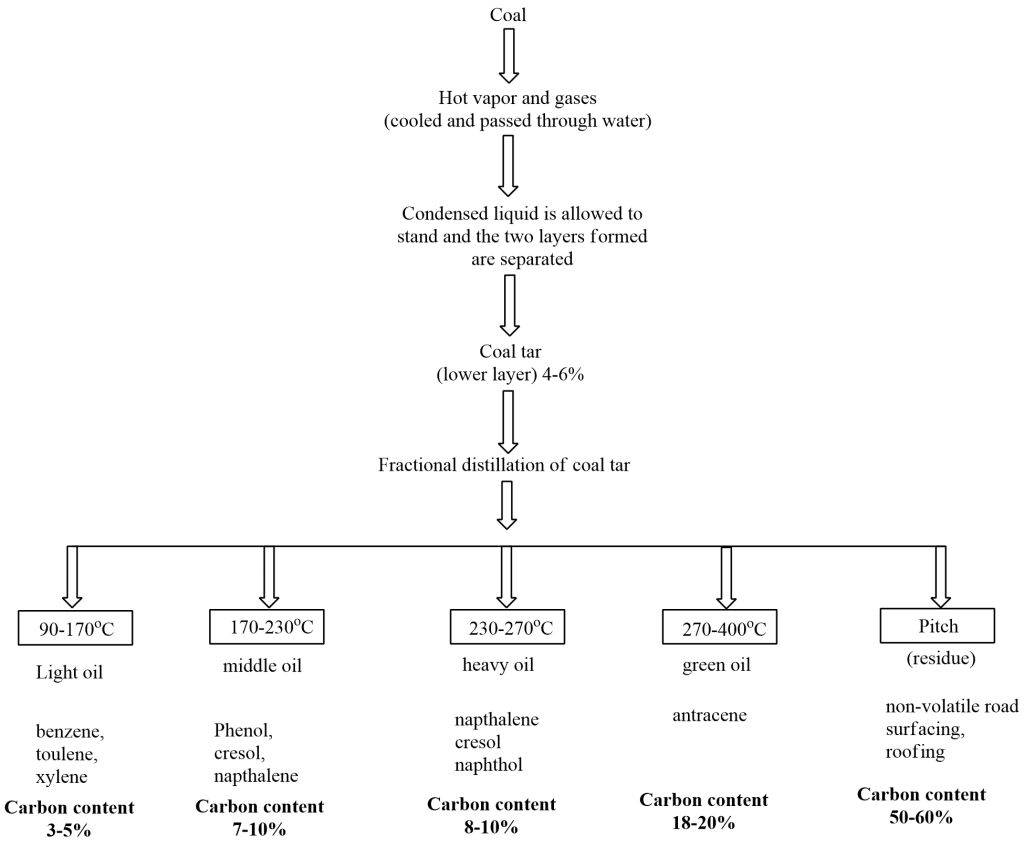
Isolation of Aromatic Hydrocarbons from coal
Coal is subjected to the following stepwise treatment for obtaining various aromatic hydrocarbons.