Table of Contents
ToggleThe stability of alkenes depends on various factors. The heat of hydrogenation can frequently provide useful information about the relative stabilities of unsaturated compounds. For example, of the isomeric 2-butenes, the cis isomer has a heat of hydrogenation of 28.6 kcal, and the trans isomer has 27.6 kcal. Both reactions consume one mole of hydrogen and yield a similar product, n-butane. Therefore, if the trans isomer evolves with 1 kcal less energy than the cis isomers, it can only mean that it contains 1 kcal less energy; in other words, the trans isomers are more stable by 1 kcal than the cis isomers.
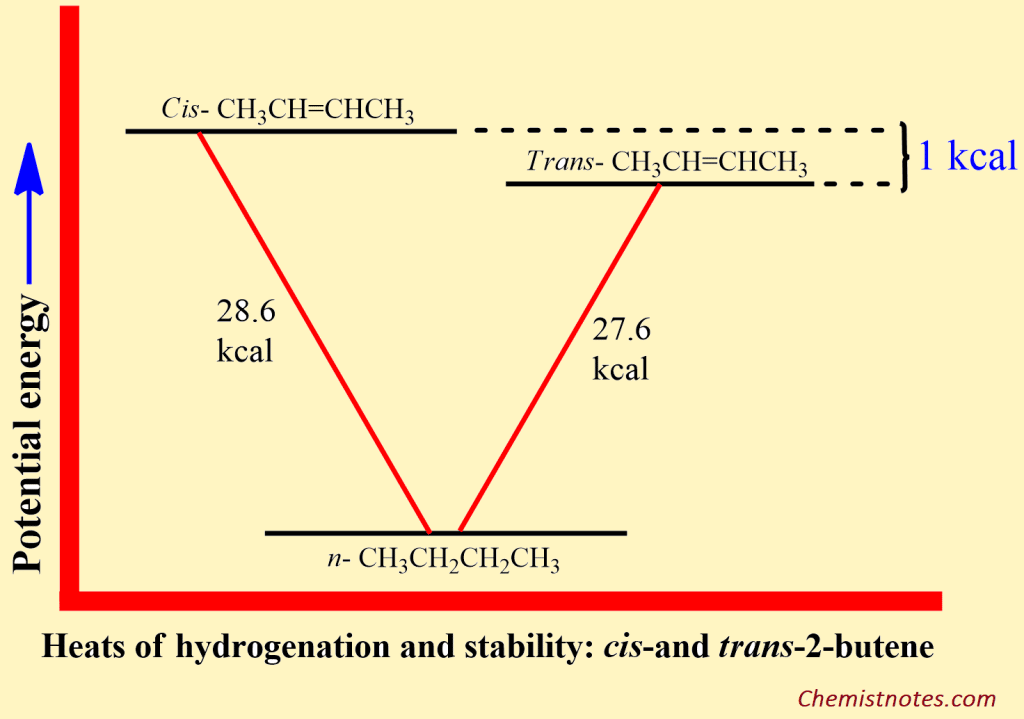
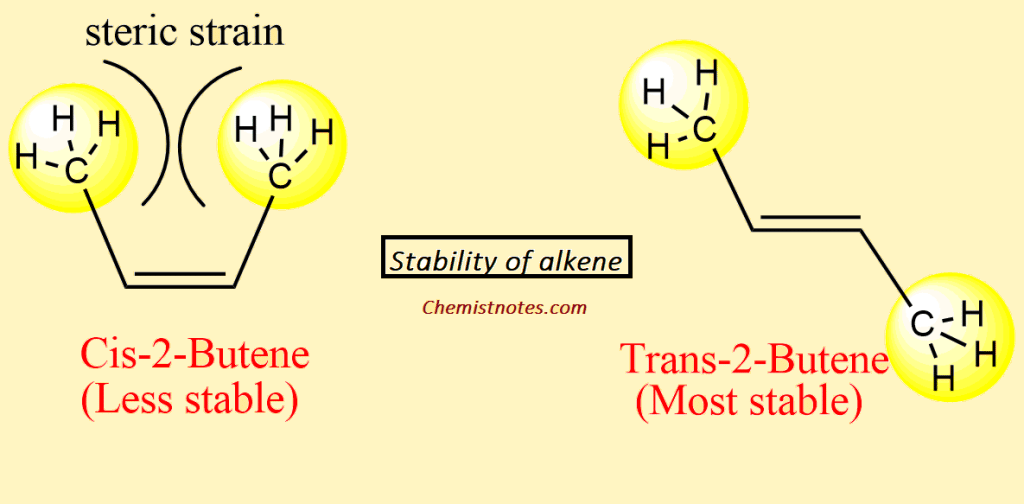
Orientation (Cis/Trans Isomers)
In comparison between cis and trans isomers, trans isomers are more stable than cis isomers. The molecular crowding in the Cis isomer led to nonbonding interaction between two alkyl groups that occupied the same side of the double bond. As a result of the crowding, steric strain develops which causes distorting bond angles and decreases the effectiveness of bond orbital overlap, and, destabilizing the molecule. Cis isomers have a higher boiling point because they have greater intermolecular forces of attraction and have higher dipole moments of the molecules.
Stability of Alkenes Increases With Increasing Substitution
The heat of hydrogenation shows that the stability of alkene also depends upon the position of the double bond. The stability of alkene depends upon the number of alkyl groups attached to the doubly bonded carbon atoms. The higher the number of alkyl groups around double-bonded carbon, the more the stability of alkene.
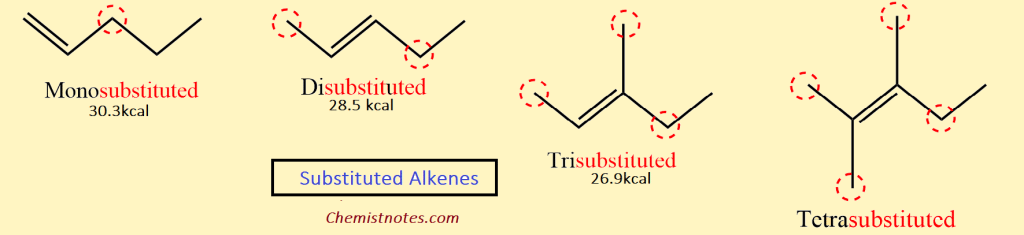
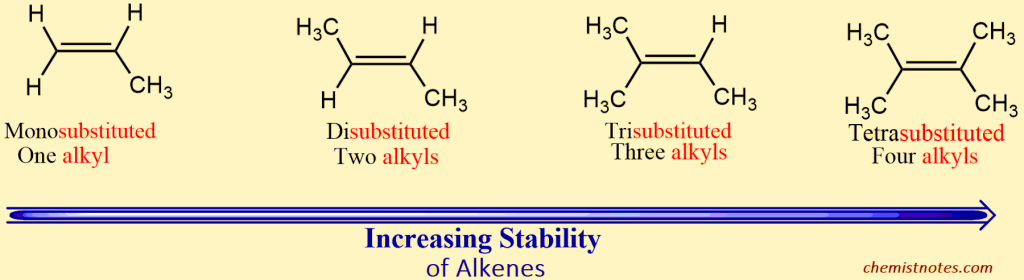
Thus, a tetra-substituted alkene is more stable than a tri-substituted alkene, which is more-stable than a di-substituted alkene or an unsubstituted one; the stability of various alkenes is shown below:
For example, the elimination reaction of 2-chlorobutane gives 2-butene as a major product. The orientation of elimination is governed by Saytzeff orientation.
FAQs
Which isomer of alkene is more stable?
Trans alkene is more stable than that cis alkene.






