Table of Contents
ToggleCrown ethers are an interesting family of complexing agents synthesized by Pedersen in 1967. The name crown ethers refer that these polyethers have a structure like crowns. Crown ethers are widely used as solvents in a variety of organic and organometallic reactions. They also return back compounds like KMnO4 and KOH soluble in benzene or other aromatic hydrocarbons.
Examples of crown ether
An example is dibenzo-18-crown-6 and the name indicates that there are two benzene rings in the compound, 18 atoms make up a crown-shaped ring, and six of the ring atoms are oxygen.
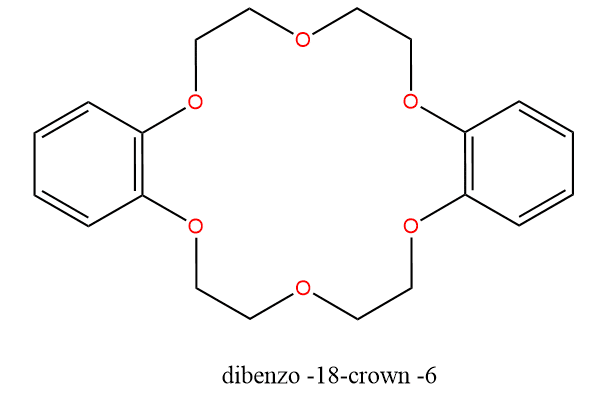
These six oxygen atoms may form complexes with a metal ion, even with large ions like Group I ions that are not very good at forming complexes. The organic part of the molecule is puckered to give the crown arrangement, and the oxygen atoms with their lone pairs are nearly planar about the metal ion at the center of the ring. The bonding of the metal ion to polyether is largely electrostatic, and a close fit between the size of the metal ion and the size of the hole in the center of the polyether is essential.
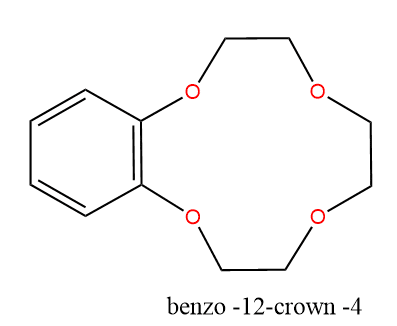
Cyclic polyethers can have varying sizes of rings; for example, benzo-12-crown-4 has a ring of 12 atoms, four of which are oxygen.
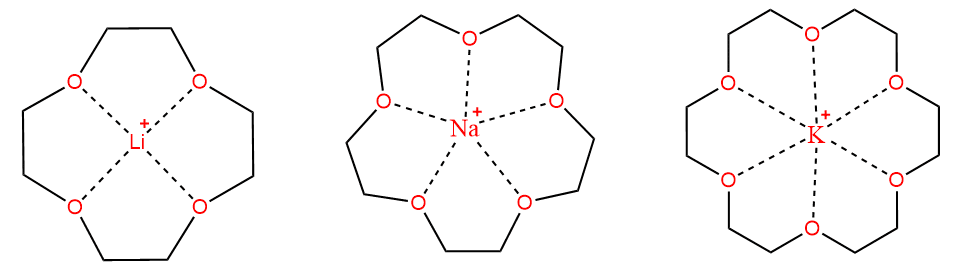
The polyether forms complexes selectively with the alkali metal ions. The size of the ring opening in the crown determines the size of the metal ions which may be accommodated. Thus, a crown-4 (a cyclic polyether with four oxygens) is selective for Li+, Na+ prefers crown-5, and K+ prefers crown-6. Crown ethers form a number of crystalline complexes, but more importantly, they are sometimes added to organic solvents to make them dissolve inorganic salts which, being ionic, would not normally dissolve. Polyethers of this type act as ion carriers inside living cells to transport ions across cell membranes, and thus maintain the balance between Na+ and K+ inside and outside cells.
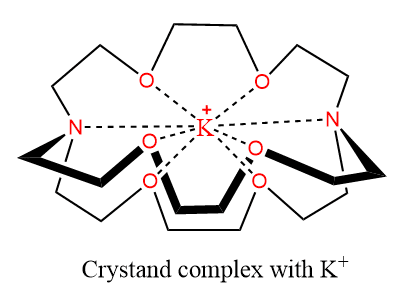
Cryptands
The cryptates are three-dimensional equivalents of the crown ethers but contain nitrogen atoms which provide branching and act as extra donor sites in addition to the oxygen atoms to bond to the metal ion. They are called cryptates because they are round shapes and hide the cation. Cryptands are used in solvent extraction. A typical crypt is a molecule N[CH2CH2OCH2CH2OCH2CH2]3N.
Examples of Cryptands
Cryptand-222 is an example of cryptands and forms a complex [Rb(crypt)]CNS.H2O in which six oxygen atoms and two nitrogen atoms in the crypt molecule bond to the metal ion, giving the metal ion a coordination number of 8.
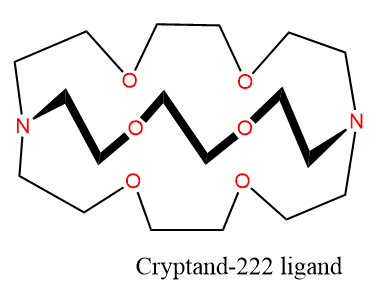
Cryptand-222 can form an unusual complex compound [Na(crptand-222)]+ Na–. This is golden-yellow in color and stable below -10oC. Here, Na+ and Na– (sodide ion) do not combine due to the shielding effect to Na+ by a large crypt ligand. Similarly, complexes containing K– (potasside), Rb– (rubidide) and caecide (Cs–) can also be formed by cooling a solution of these metals in ethylamine with cryptand-222.