Table of Contents
ToggleBeer Lambert law is the combined form of Beer’s law and lambert’s law. This law relates the intensity of light absorbed with the thickness of the absorbing medium and the concentration of the solution.
Beer lambert law definition
According to Beer lambert law, ” when a beam of monochromatic light is passed through a solution of an absorbing substance then the rate of decrease in intensity of radiation with the thickness of absorbing solution is directly proportional to intensity as well as to the concentration of the solution.”
Beer lambert law derivation
Mathematically, Beer lambert law can be expressed as:
-dI/db ∝ IC
or, -dI/db = KIC ………………………………..(i)
where I=Intensitiy of light at any thickness b
C= concentration of solution
dI=Infinitesimally small decrease in intensity of radiation
db= infinitesimally small decrease in thickness b
K= proportionality constant called molar coefficient whose value depends upon the nature of absorbing medium.
On rearrangement of equation (i), we get,
-dI/I=KCdb
If Io is the intensity of incident radiation when b=0, then the intensity of radiation It after passing through any finite thickness b of the medium can be obtained by integrating the above equation.
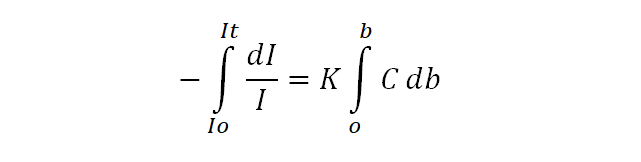
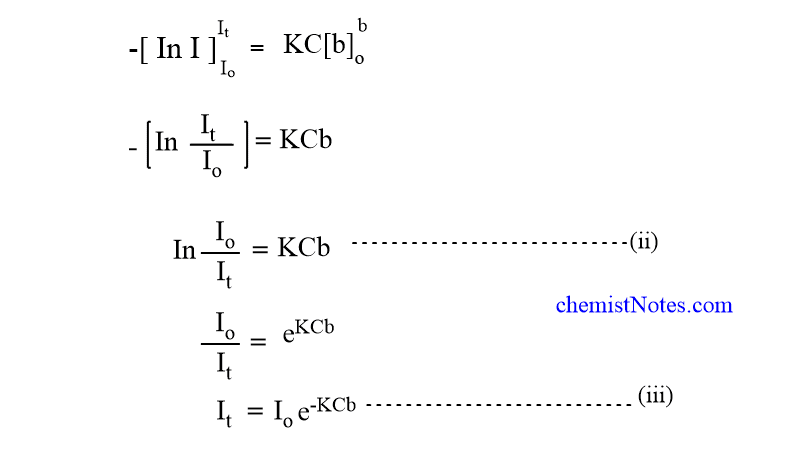
Equation (iii) shows that the intensity of a monochromatic beam of light decreases exponentially when the thickness of the medium and intensity increase. Changing natural logarithm to the base 10, equation (ii) becomes,
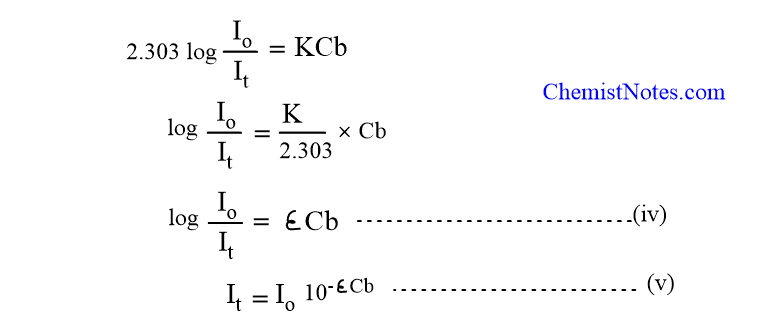
Where, ε = K/2.303, is known as the molar extinction coefficient of the absorbing medium.
From equation(iv),
log(Io/It) = εCb
The term log(Io/It) is called absorbance(A). Absorbance is a dimensionless quantity that depends on the path length, the concentration of absorbing substance, and the wavelength of light. Hence,
A=εCb……………………………(vi)
When C= 1 mol dm-3 and b=1 cm then, A=ε.
Hence, the molar extinction coefficient(ε) can be defined as absorbance for a concentration of 1 mol dm-3 and a path length of 1 cm.
The ratio (It/Io) is called Transmittance(T). Now, relate absorbance(A) and Transmittance(T).
A=log(Io/It)
A=log(1/T)
A=-log( T ) ………………………………(vii)
Hence, the absorbance is equal to the negative logarithm of transmittance.
Verification of Beer lambert law
From Beer Lambert’s law, we have
A=εCb
This law can be verified by plotting the absorbance(A) against the molar concentration (C) of the solution. A straight line passing through the origin and having a slope εb is obtained.
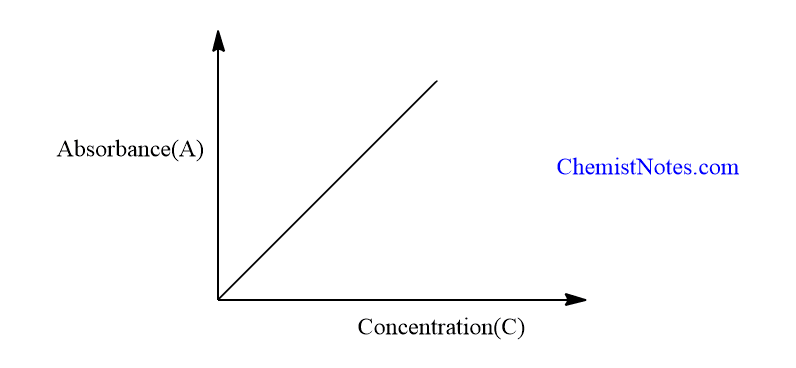
The deviation from a straight line is observed in some cases due to the dissociation, association, or ionization of the absorbing species in the solution.
Application of beer lambert law
Some applications of beer lambert law are given as:
- The concentration of the unknown solution can be determined by comparison with a solution of known concentration using a uv spectrophotometer, the principle is based on Beer-Lambert Law.
- The purity of a substance can be checked by the measurement of the absorbance of a compound by using a spectrophotometer.
- The principle of Uv visible spectroscopy is based on this law.
Limitations of beer lambert law
The limitations of Beer-lambert law are given as:
- Beer-Lambert law is only valid on monochromatic light
- This law is applicable under a low concentration range where interactions between molecules are not considered.
- This law is also invalid when radiations of very high intensities are used.
Deviation of beer lambert law
When a plot of absorbance as a function of concentration at a particular path and wavelength of monochromatic is drawn, a straight line passing through the origin is obtained. But when concentration is very high, a plot of absorbance and concentration deviates from linear behavior. The main causes of such deviation from Beer Lambert’s law are given as:
- The deviation may occur when the light of a single wavelength is not used
- Polymerization of solutes during the measurements
- Association, dissociation, or ionization of solutes causes deviation
- Presence of some other substance that absorbs at the same wavelength as the solute may cause deviation.
- The presence of some impurities in the colored compounds may cause deviation
References
Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012






