Table of Contents
ToggleWhat is an alkene?
Alkenes are hydrocarbons that contain a carbon-carbon double bond (=C=C=) in their molecule. Alkenes are commonly known as olefins (in Latin, olefins mean “oil-forming) because the lower member of the series reacts with chlorine to form oily products. Ethylene (C2H4) is the first member of the series. The carbon-carbon double bond is known as the olefinic linkage or the ethylenic linkage after the name of the first member.
Alkene formula
They have the general formula CnH2n (n=number of carbon atoms). Alkenes contain two fewer hydrogen atoms than alkanes (CnH2n+2) and are thus known as saturated hydrocarbons. Following are some examples of alkenes.
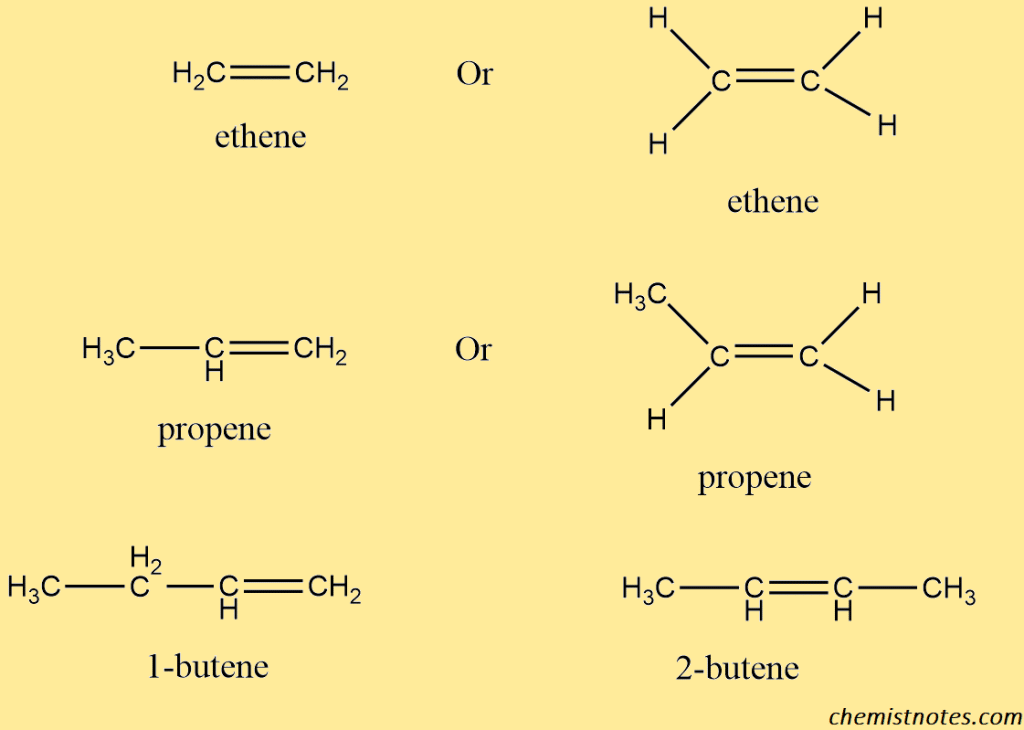
Structure of alkenes
The alkenes are more reactive than the alkanes because it contains an extra π-bond between carbon atoms than in alkane. In alkenes, the doubly bonded carbon undergoes sp2 hybridization. The sp2 hybridization in alkenes can be explained by taking the formation of an ethene molecule as an example.
The electronic configuration of carbon atom is:
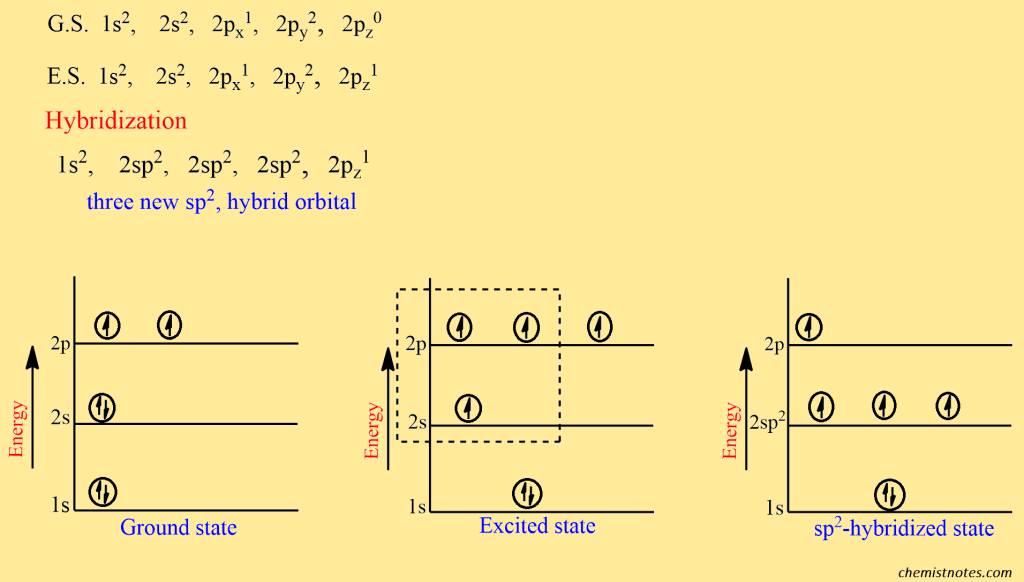
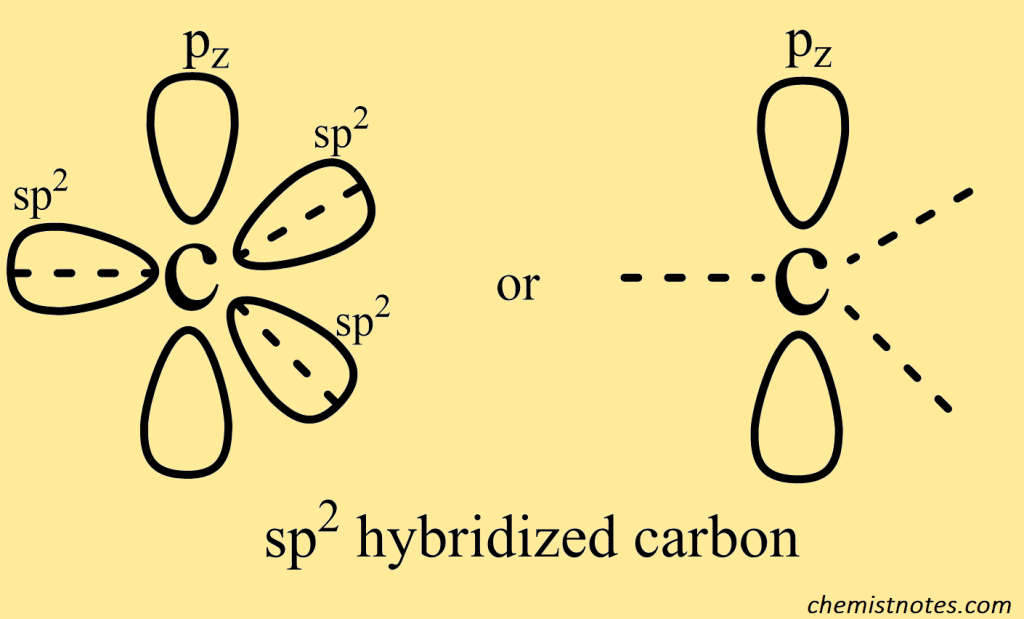
The sp2 orbitals above are identical (same energy and shape) but differ only in their orientation in space with respect to each other. The three sp2 orbitals lie in the same plane with their axes towards the corners of an equilateral triangle. The angle between any pair of orbitals is thus 120o. The unhybridized pz orbital is oriented along an axis perpendicular to the plane of sp2 orbitals, with each lobe above and below the plane.
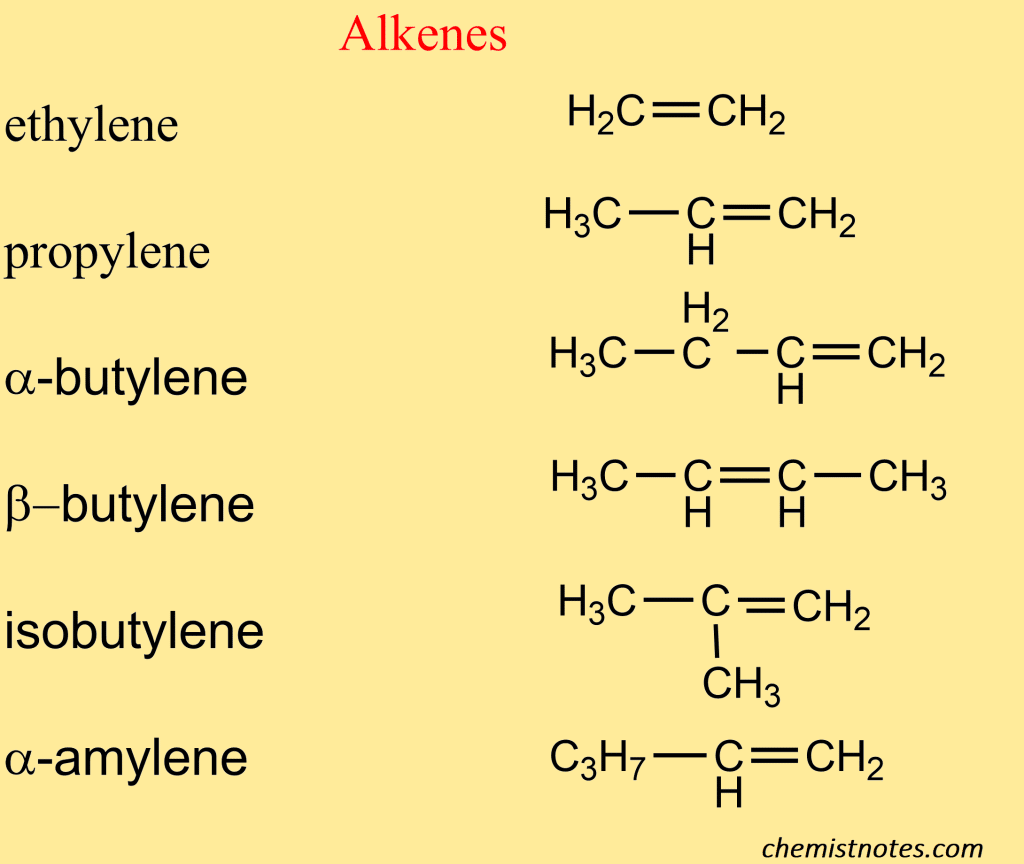
Naming (Nomenclature) of alkene
There are two ways of naming alkenes:
Common name
The common names of the first few alkenes are derived from the corresponding alkanes by replacement of ‘ane’ of the common name of saturated hydrocarbon by ‘ylene’.
IUPAC system
In this system ending ‘ane; of the corresponding alkane is replaced by ‘ene’. The series is thus known as the alkene series. The name of the hydrocarbon is based on the parent alkene having the longest carbon chain containing a double bond and its position is indicated by the number of a carbon atoms at which the double bond is originate. The name of the parent alkene with the position number of the double bond is written first and then the names of the other substituent prefixed to it.
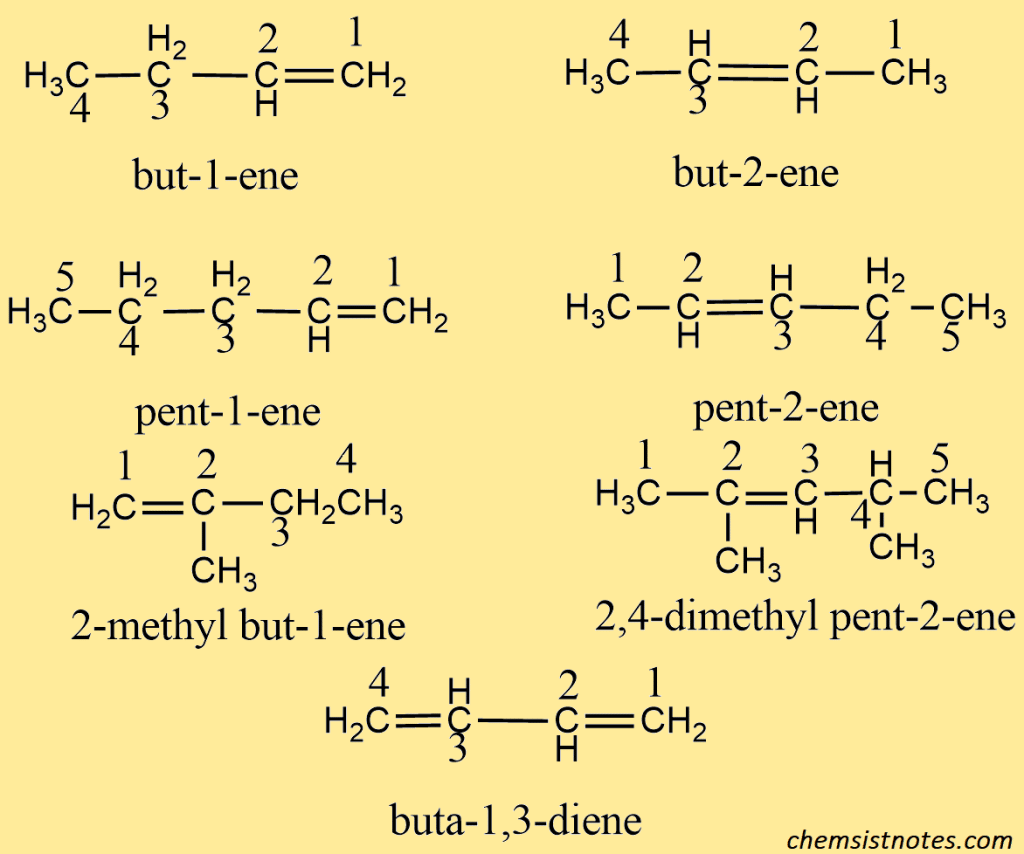
When there are two or three double bonds in a molecule, the ending -ane of the corresponding alkane is replaced by ‘-adiene’ or ‘atriene’ to get the name of the alkene.
Physical properties of Alkene
- First, three members of the alkene series ( ethene propene and butene) are gases at room temperature while alkene having five to fifteen carbon atoms (pentene to pentadecene) are liquid and higher and higher and higher alkenes are solid.
- Alkene is insoluble in water but they are soluble in non-polar organic solvents.
- They have characteristics of smell and burn with a luminous flame.
- They are slightly more polar than the corresponding alkene due to the presence of sp2 hybridized carbon atoms. The unsymmetrical alkene has a small dipole moment.
- They are less volatile than alkenes.
- The boiling point, melting point, and specific gravity of alkene, in general rises with an increase in molecular weight in homologous series.
| Name | boiling point | melting point | SP. gravity |
| ethene | -102 | -169 | – |
| propene | -48 | -185 | 0.514 |
| 1-butene | -6.5 | -185 | 0.595 |
| cis-2-butene | 4 | -139 | 0.621 |
| trans-2-butene | 1 | -106 | 0.604 |
| 1-pentene | 30 | −165.2 °C |
Uses of alkene
- Ethane -1 2-diol is prepared by alkenes, which is an anti-freezing agent used in motor car radiators.
- Alkene is used to make polymers like polythene, which is used to make bowls, containers, bags, and other items.
- The manufacture of polystyrene is used to create components for refrigerators and battery cases for automobiles.
- Alkenes are used in the production of acrylic fibers.
- Alkenes are used to make synthetic fibers and ethanol or drinking alcohol, respectively.
- Alkenes are used to prepare propanol, propanol is used to prepare acetone, commonly known as nail polish remover.






