Table of Contents
ToggleThe determination of configuration of cis and trans isomers can be done by various methods, which have been discussed below. Before going to determination methods, let’s know basic things about geometrical isomers.
Simple way to understand geometrical isomers:
Compounds with the same empirical formula but different atom arrangements are referred to as isomers. The orientation of a functional group in a compound is described by a geometric isomer. In a geometric isomer, the atoms are often joined by a double bond that does not rotate, but it is possible that the ring structure may also play a role. The isomers are given the names cis and trans depending on where the functional group is located.
How to differentiate cis and trans isomers?
The geometrical isomers can be differentiated mainly by two methods.
- Physical methods
- Chemical methods
Physical methods
Following are some physical methods to determine the configuration of cis and trans isomers.
- Dipole moment
- Uv-visible spectra
- NMR
- Melting and boiling point
- Acid strength
- X-ray diffraction method
1. Determination of cis and trans isomers by the dipole moment
As we are simply familiar with the term dipole moment, it is the difference in electronegativity between two atoms. For example, the electronegativity difference between hydrogen (2.2) and fluorine( 4.0). It can be simply calculated as μ= e × d, where e represents the charge and d is the separation between the charges.
let us take one simple example to understand the dipole moment relationship between cis and trans. Example XCH=CHY, if X and Y both are electron-withdrawing groups they show -I effect and show polarity in the case of cis isomers while in trans no polarity is observed due to dispersion of charge in one direction hence the dipole moment of cis is higher than trans.
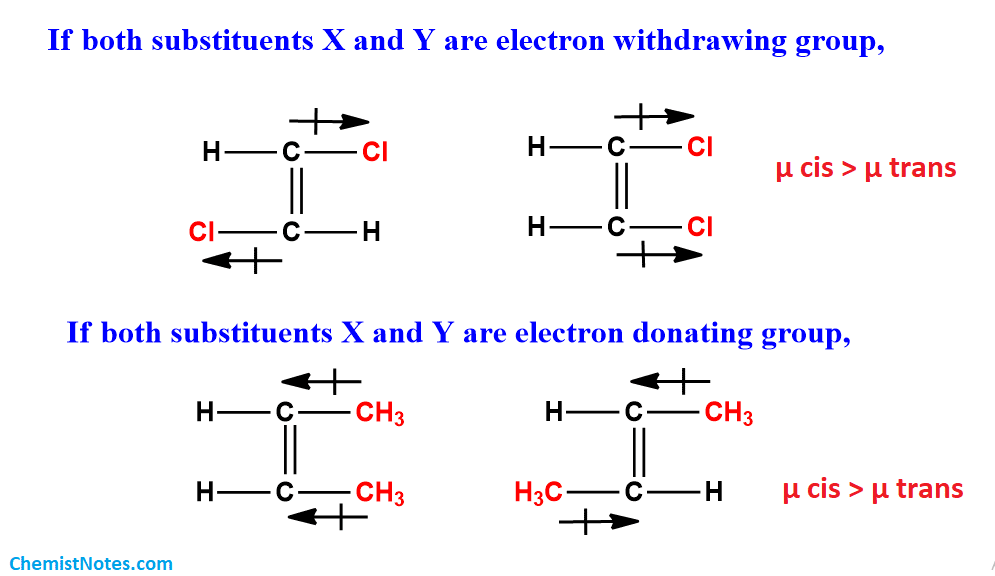
But in the case of if one substituent is electron-withdrawing and another one is electron releasing then due to the polarity difference, the dipole moment of trans is higher than cis.
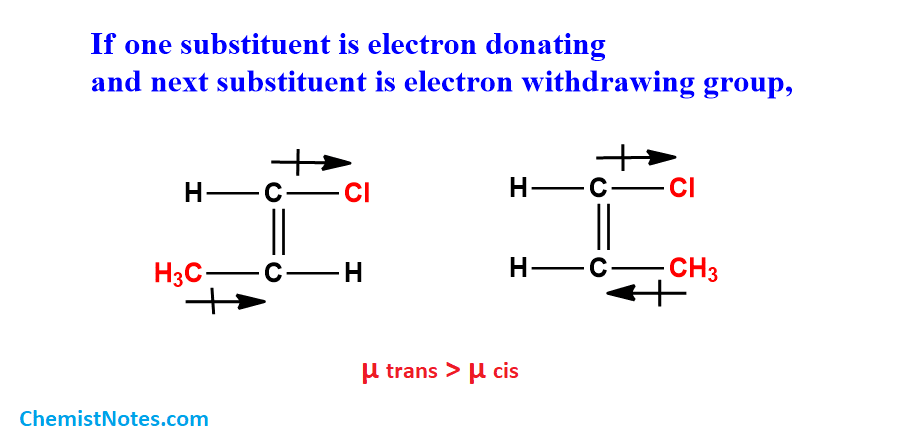
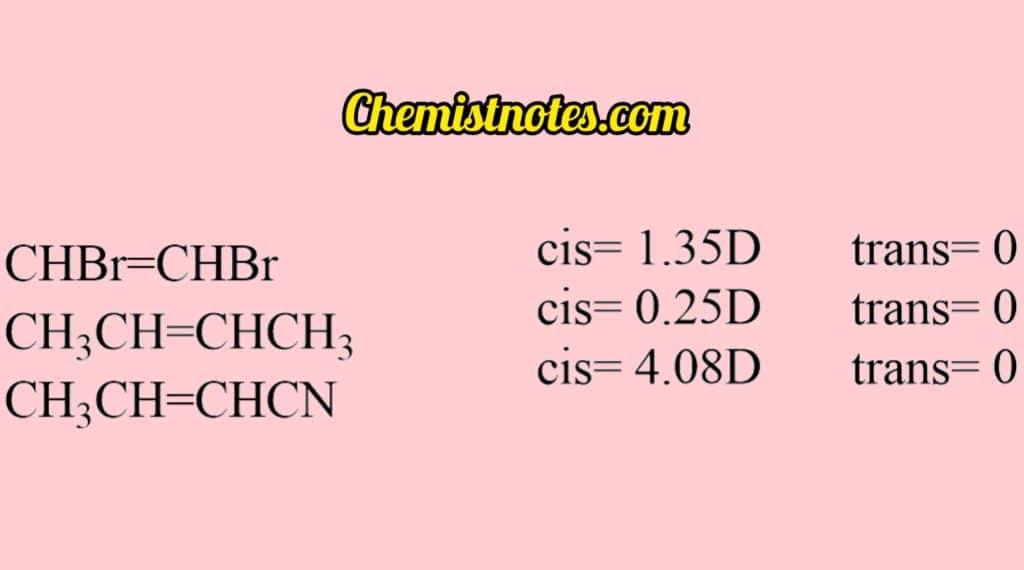
In the case of tri-substituents XCH=YCZ, then the dipole moment depends upon the disposition of substituents.
2. Determination of cis and trans isomers by using melting and boiling points
Trans isomers have higher melting points and lower boiling points than cis isomers generally because they need more energy and have greater symmetry. Since isomers with larger dipole moments are strongly attracted to one another by electrostatic interactions between their oppositely charged ends, trans isomers are thought to have lower boiling points and higher melting points than cis isomers.
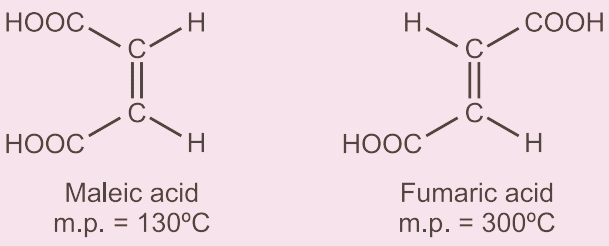
For example, in the case of maleic acid ( cis) and fumaric acid ( trans) the melting point of trans is higher than cis and likewise boiling point of cis is higher than trans isomers. Maleic acid( 130°C) and fumaric acid (300°C).
3. By using Uv- visible spectroscopy
By measuring the wavelength and absorbance, ultraviolet-visible spectroscopy may also distinguish between cis and trans isomers. Trans isomers are observed in shorter wavelengths and found in the UV region than cis isomers because they are more symmetrical and require more energy for the transition than cis.
But in the case of stilbene, the case is just reversed. The trans-stilbene has a longer wavelength while cis has the shorter one. This is due to the structure of trans stilbene which is coplanar and their resonance between ethene and benzene rings is maximum but in the case of cis, there is a steric factor that forces one of the benzene rings out of the plane, and needs more energy and observed in shorter wavelengths.
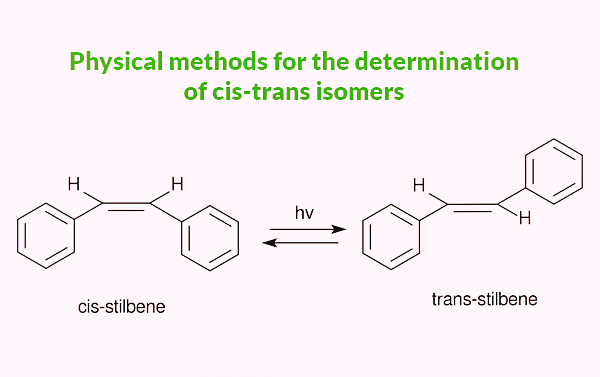
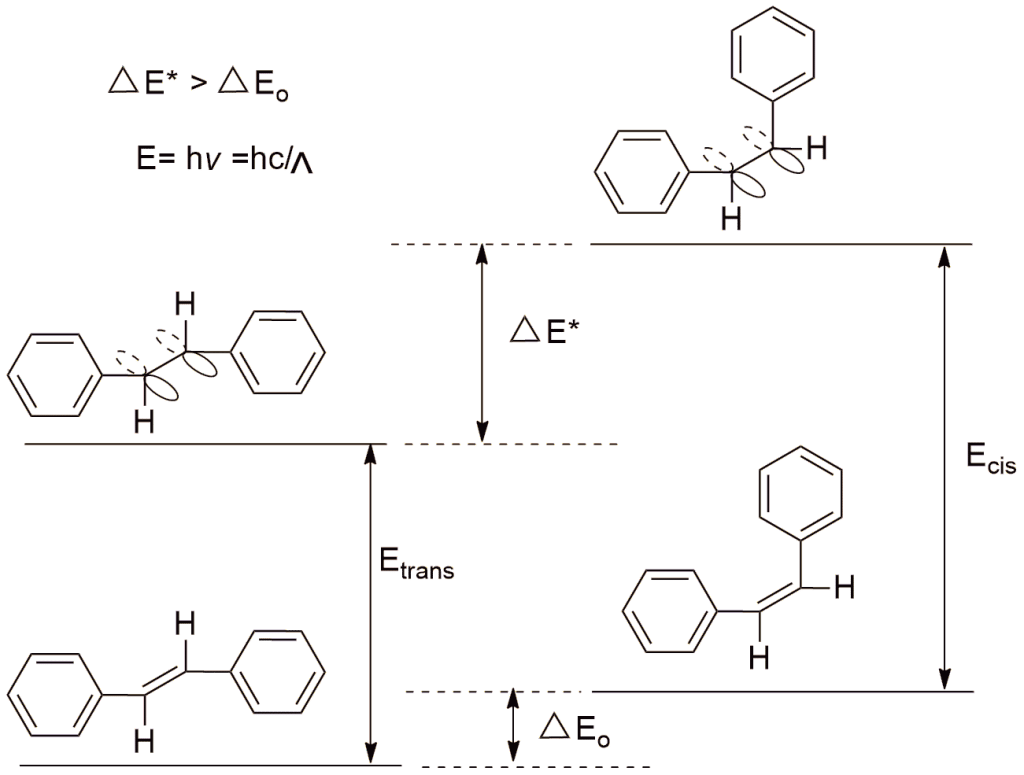
4. By using NMR spectroscopy
The most effective and adaptable method for identifying cis and trans isomers is NMR. Nuclear magnetic resonance (NMR) is a very sophisticated technology that allows for the determination of the total number of hydrogen atoms in a molecule as well as the number of carbon atoms in the molecule. Generally, In NMR spectroscopy we focus on chemical shift (ppm) and the cis isomers go to the shielded region ( upfield) whereas trans goes to deshielded side ( downfield).
5. By comparing Acidic strength
Acid strength is dependent on resonance and the Pka values of certain substances. Since cis has a lower Pka value than trans, and since hydrogen concentration and Pka value are inversely associated, cis isomers have a higher acid strength. For instance, fumaric acid has a Pka value of 4.70 while that of maleic acid is 4.4, meaning that it is a stronger acid.
6. By using X-ray diffraction method
The most effective method for precise structural characterization of crystalline substances is single-crystal X-ray diffraction. It displays the spatial atomic arrangement and creates a picture of the crystal’s interior structure. The primary source of data on the geometrical structure of molecules, including bond lengths, bond angles, flexible molecule conformations, and intermolecular interactions, is single-crystal X-ray diffraction.
FAQs/MCQs:
1. What are the necessary two conditions to be cis and trans isomers?
The following two conditions must be met for cis-trans isomerism: The molecule must restrict rotation. Each doubly bound carbon atom must have two dissimilar groups.
2. What are the different physical methods to determine the configuration of cis and trans isomers?
The various types of physical methods to determine the configuration of cis and trans isomers are dipole moment, NMR spectroscopy, IR, acid strength, melting and boiling point, etc
3. Which isomers have a higher boiling points?
Cis isomers have a higher boiling point.
4. How do you determine the configuration of cis and trans isomers?
The alkene is the cis isomer if the two groups are on the same side of the double bond. The alkene is the trans isomer if the two groups are on different sides of the double bond.
5. What are cis and trans isomers?
The diastereomer is referred to as cis when the substituent groups are oriented in the same direction, whereas the diastereomer is referred to as trans when the substituent groups are oriented in opposite directions. But-2-ene is an illustration of a minor hydrocarbon exhibiting cis-trans isomerism.
6. What is the wavelength value for trans and cis stilbene?
The wavelength of trans and cis stilbene are 295 and 283 nm respectively.
7. Which one has high acidic strength cis or trans isomers?
Cis isomers have higher acidic strength.
8. Which isomers are more shielded?
Cis isomers are observed in the shielded side and are upfield.






