Table of Contents
ToggleWoodward Fieser rule, devised by Woodward and Fieser, is an empirical correlation of structural variation that helps us to predict the wavelength(λmax) at which a conjugated diene, triene, will absorb. In simple words, the position of the most intense Uv-vis absorbance peak/band can be predicted with the substituents present in the conjugated diene or triene.Therefore, it is one of the important emprical rule in UV visible spectroscopy.
Commonly used terms in the Woodward-Fieser rule
Before moving to the rule, it’s mandatory to know some terms so that it would be easy to apply the rule to predict the position of absorbance.
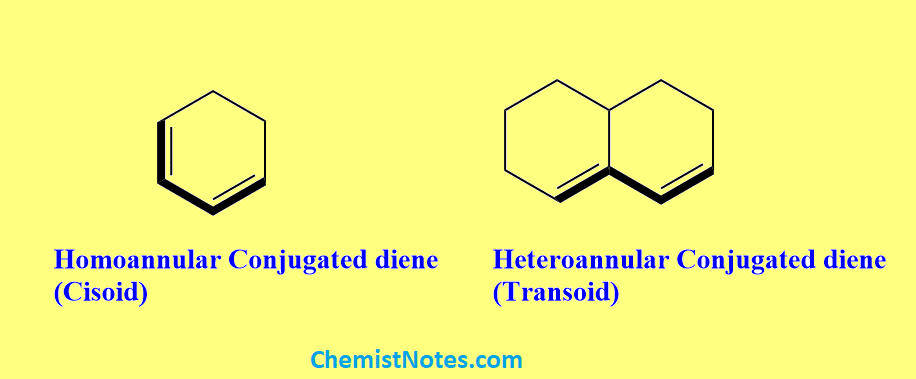
Homoannular conjugated diene
If the conjugated double bonds are present in the same ring of cyclic diene, the diene is known as a homoannular conjugated diene. These are also known as Cisoid and according to the Woodward-Fieser rule, their base value is taken as 253 nm.
Heteroannular conjugated diene
If the conjugated double bonds are present in the two different rings, the diene is known as a heteroannular conjugated diene. These are also known as Transoid and their base value is taken as 214 nm.
What is Base value? The base value or parent value is the wavelength(Lamda max) at which the unsubstituted normal diene absorbs the Uv-vis radiation.
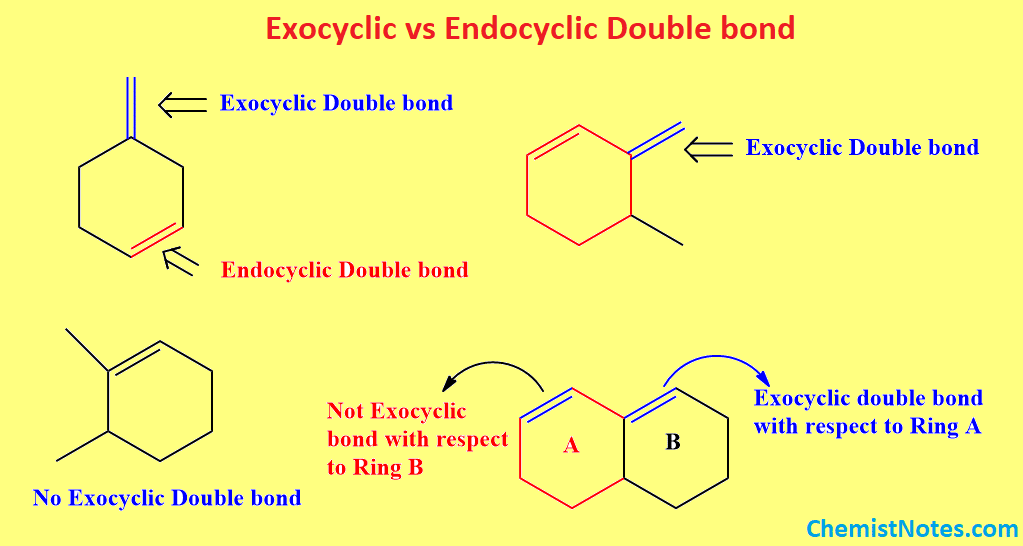
Exocyclic double bond
If both carbon atoms connected by a double bond are not the members of the same ring, it means only one carbon is the member of the ring, then the double bond is said to be an exocyclic bond. In other words, it is that double bond, which is present just outside of a cyclic ring as shown below.
Endocyclic double bond
If both carbon atoms connected by a double bond are members of the same ring, then the double bond is said to be an endocyclic double bond. In other words, it is that double bond, which is present within a cyclic ring as shown below.
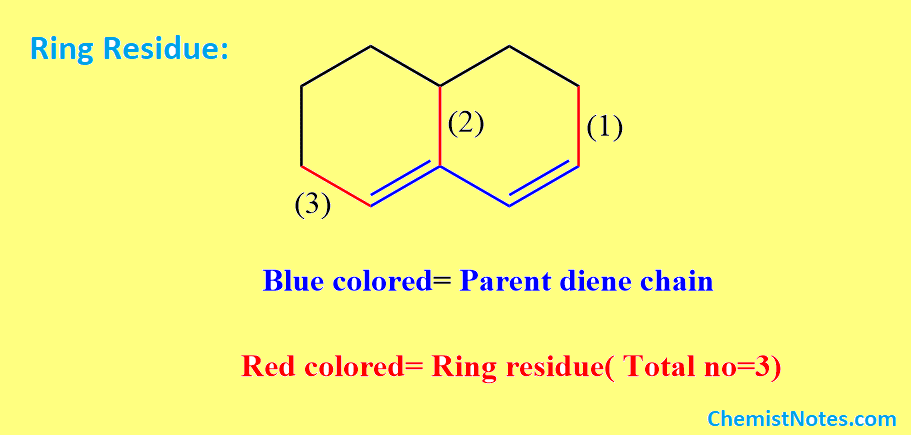
what is ring residue in Woodward-Fieser rule?
Ring residues are substituents that are attached to the parent chain of a conjugated diene or the extended chain of a conjugated diene. The parent chain only extends when there is a suitably located double bond participating in the conjugation with the parent chain.
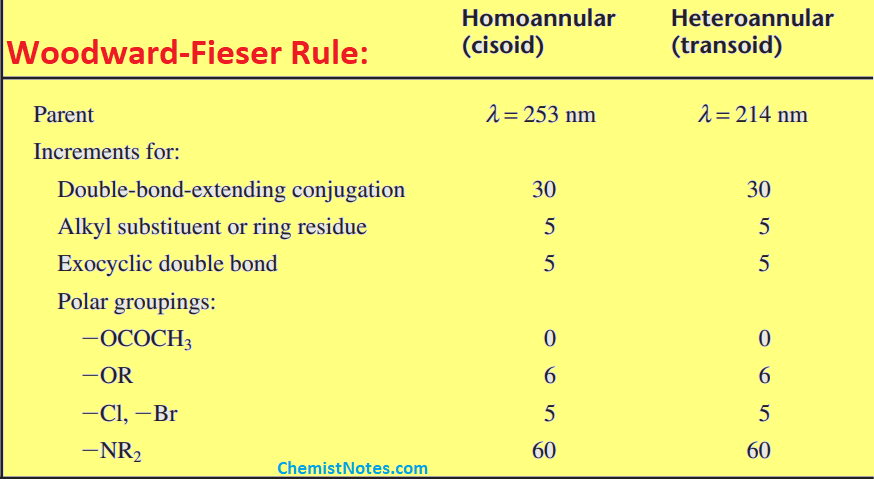
Woodward Fieser rule for conjugated dienes
In order to apply this rule, First of all, the correct base value of conjugated diene is chosen and the contributions made by each substituent are added to the base value.
The basic steps or tricks to apply the Woodward-Fieser rule are explained below.
- Identify conjugated diene: The first step is to identify which type of conjugated diene is given i.e. notice it’s either homoannular(Cisoid) or heteroannular(Transoid) conjugated diene. As mentioned earlier, the base value of cisoid and transoid are 253nm and 214nm respectively.
- Is there any double bond extending conjugation?: If there are extra double bonds that extend the conjugation, note down the number of the double bonds extending the conjugation. According to Woodward fieser rule, the increment for double bond extending conjugation is 30 nm. It means each such bond contributes 30 nm increments to the base value for both cisoid and transoid.
- Count the total number of alkyl substitutions or ring residues: Note down the total number of alkyl substitutions and ring residues (described above). Each such alkyl substitution or ring residue contributes 5 nm increments to the base value for both cisoid and transoid.
- Identify exocyclic bond: If there are exocyclic bonds in the system, note down the total numbers of them, and each such bond contributes 5 nm increments for both cisoid and transoid.
- Is there any auxochrome directly attached to the conjugated diene?: Groups such as -OCOCH3, -OR, -Cl, -Br, and -NR2 also contribute 0 nm, 6nm, 5nm, 5nm, and 60 nm increments respectively only if these are part of the conjugated diene, otherwise they don’t contribute.
- Add all the increments contributed by the substituents, exocyclic bond, double bond extending conjugation, and auxochromes to the base value.
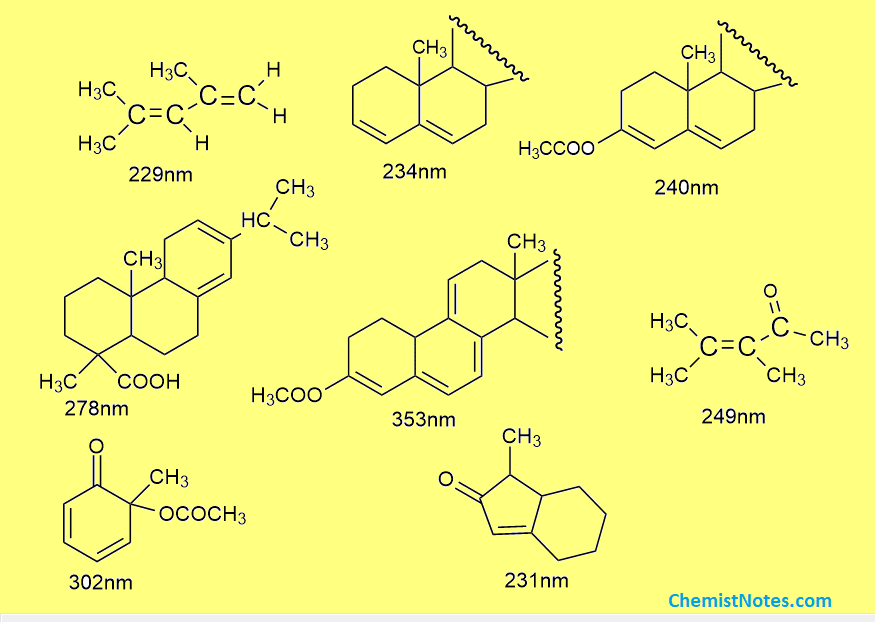
Let’s see some examples:
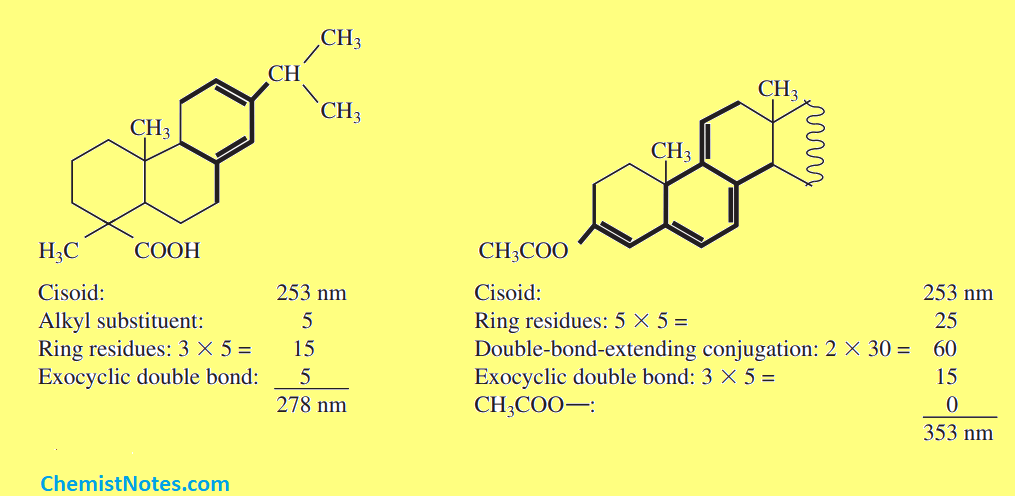
Woodward fieser rules for enones
By examining lots of Uv spectra of enones, Woodward derived a set of rules to predict the wavelength at which the unknown enone will absorb so that π to π* transition is observed. Let’s discuss these rules.
- First of all, we must know which type of enones is given. The base values of some enones are given below:
- Six-membered ring or acyclic parent enone = 215 nm
- Five-membered ring parent enone = 202 nm
- Acyclic dienone = 245 nm
2. Add increments, to the base value, contributed by double bond extending conjugation, alkyl group, or ring residue, exocyclic double bond, and homocyclic diene component.
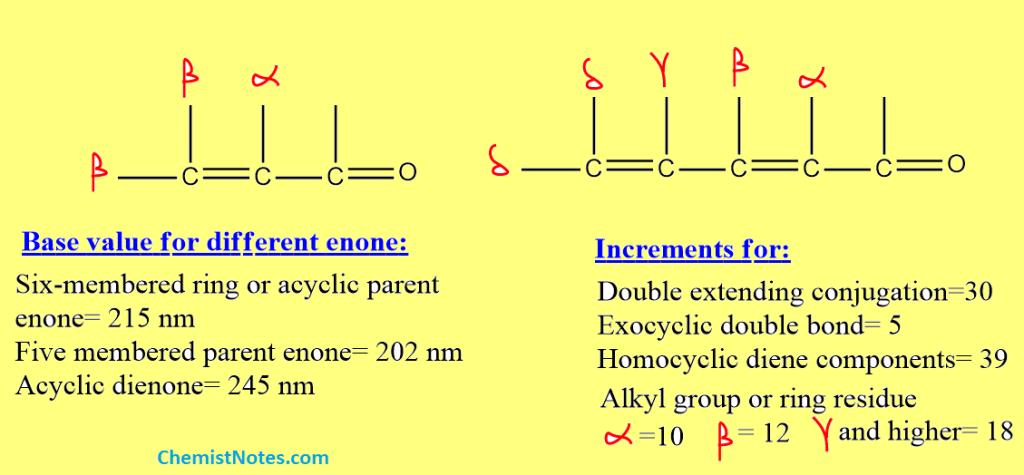
3. Similarly, Add increments to the base value contributed by the polar groups such as -OH, OCOCH3, -OCH3, -Cl, -Br, -NR2.

Let’s see some examples:
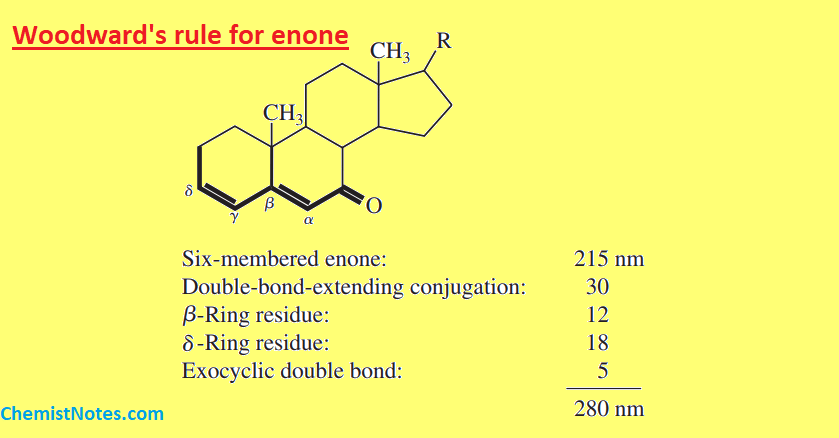
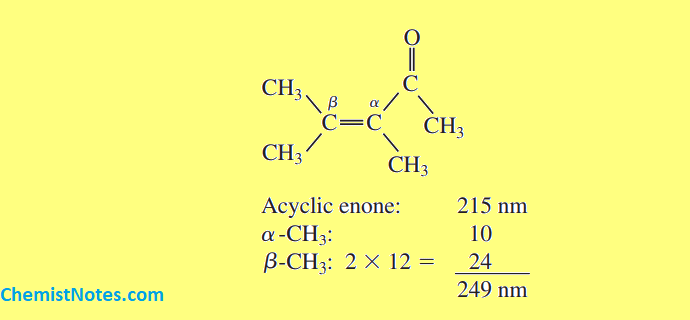
Woodward-Fieser rule application
As stated earlier, this rule is very popular to determine the position of the absorption maxima(λmax). The λmax value of conjugated diene and unsaturated ketones determined by using this rule differs slightly from the observed value but it gives a tentative idea about the position of absorption.
Limitations of Woodward Fieser rule
The Woodward rule for diene and carbonyl compounds gives reliable results only where there is an absence of strain around the chromophore. Thus, the rules are successful for acyclic and mostly six-membered ring systems. This rule is violated when the chromophore distortion is engendered by ring strain or by the introduction of additional conjugation other than at the end of the chromophore.
Woodward-Fieser rules practice problems
Here are some problems related to this rule. Try to solve these problems and practice more and more.







3 Responses
Its better
I want ylo learn mote about it…can u please aprovide any notes for me for preparing CURT chemistry entrance exam ….of mew syllabus
I want to learn more and how to go about enone under WOODWARD’S RULE