Table of Contents
ToggleCompounds having the same molecular formula but different structural arrangements are called isomers, and the phenomenon by which compounds show different chemical structures and properties though having the same chemical formula is called isomerism.
The term isomers are derived from the Greek words ‘isos’ and ‘meros’, meaning “made of the same parts.”
Types of Isomerism
There are two types of isomerism i.e. structural isomerism and stereoisomerism, which can be further divided into their subtypes.
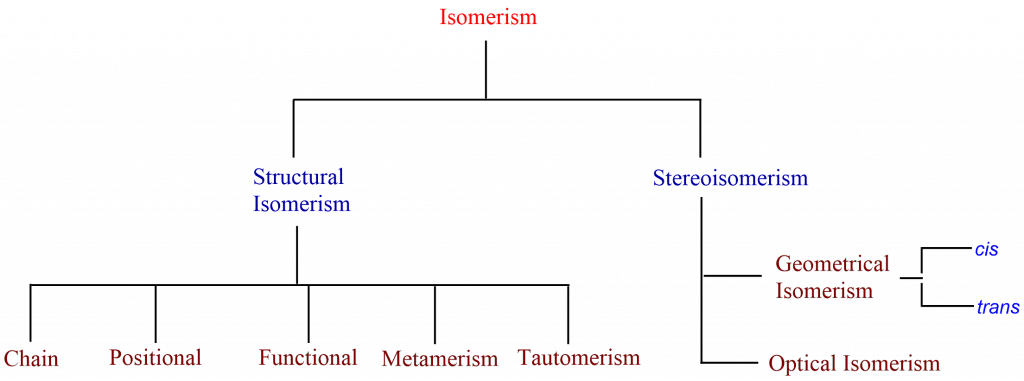
Structural Isomerism
In structural isomers, compounds have the same molecular formula but a different order of connectivity of atoms i.e. different constitutions, and hence also known as Constitutional isomers. The functional groups and atoms in these isomers’ are linked in various ways. It is sub-categorized into the following isomers.
Chain Isomerism
Here, the isomers possess the same molecular formula but have different branched structures i.e. different orders of connectivity of carbon to each other. Some of the examples of chain isomers are:
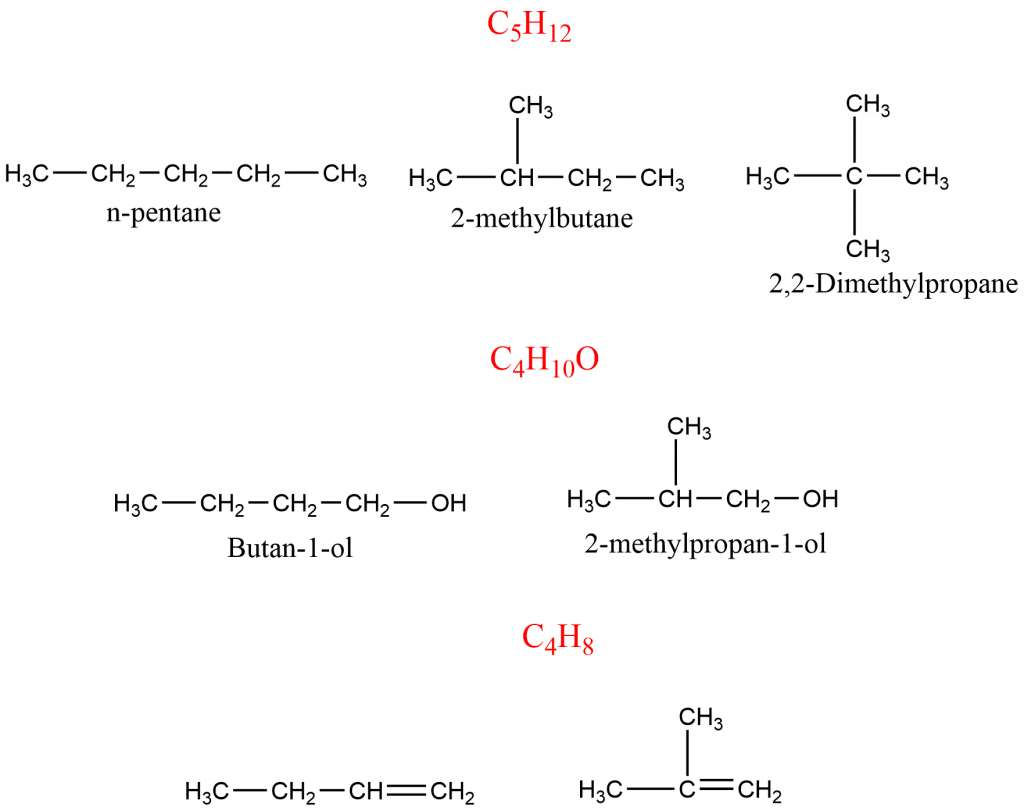
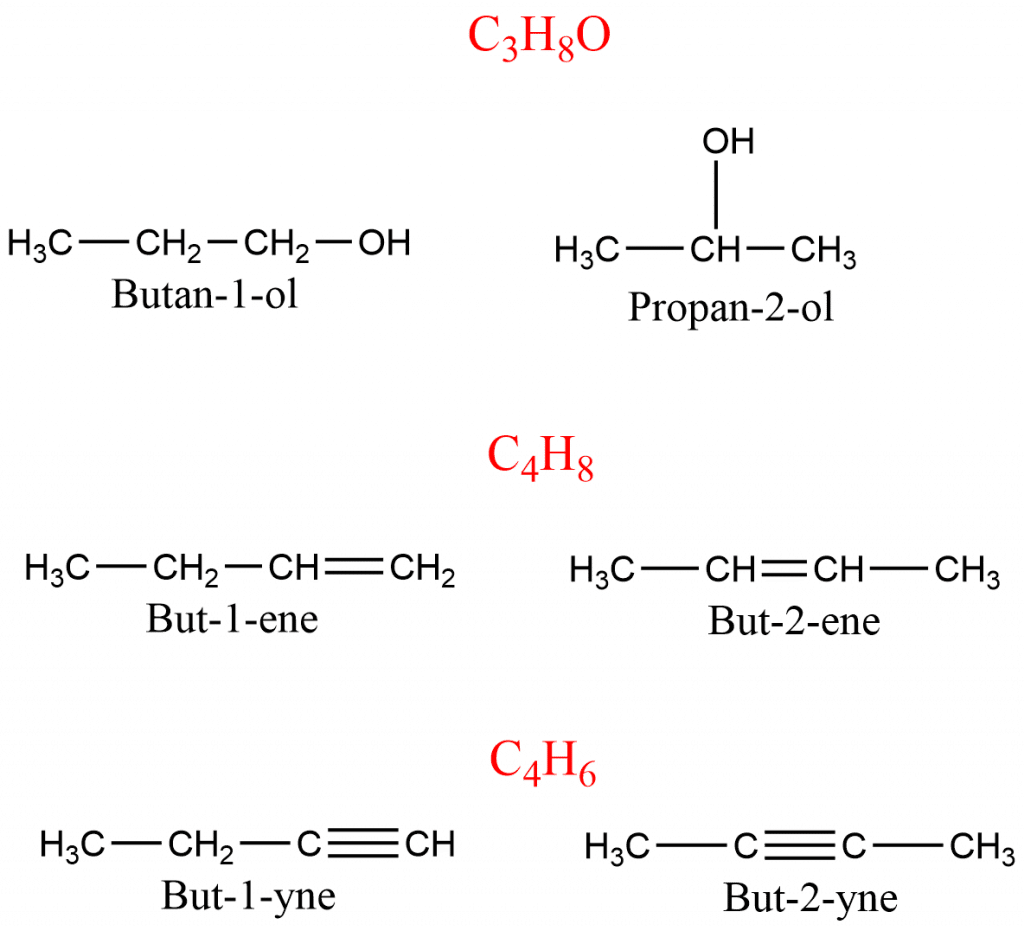
Positional Isomerism
In positional isomerism, compounds have the same carbon skeleton but they differ in the position of the functional group attached to it. The compounds having a molecular formula such as C3H8O, C4H8, C4H6 exhibit positional isomers.
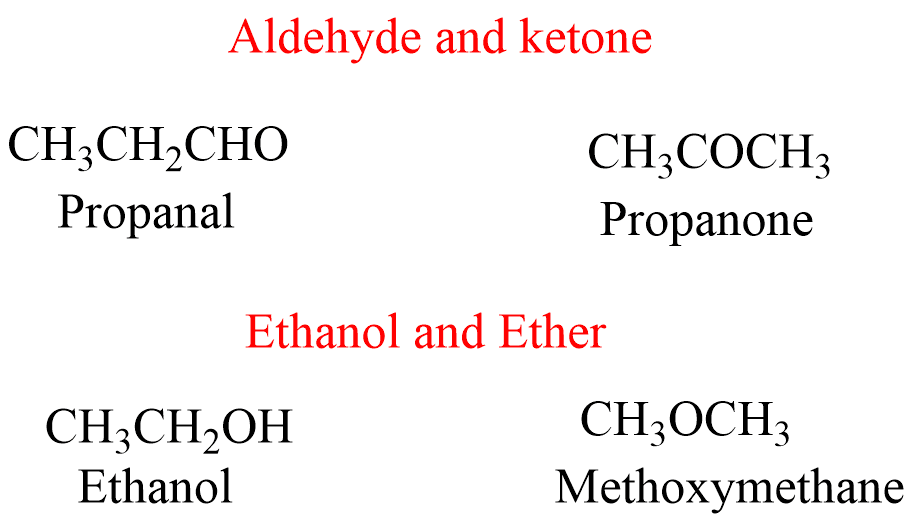
Functional Isomerism
Compounds with the same chemical formula but different functional groups attached are called functional isomers, and the phenomenon is called functional isomerism. Compounds such as ethanol and ether, aldehyde and ketone, cyanide and isocyanide, etc. are functional isomers.
Metamerism
In such isomerism, the isomers have the same chemical formula but differ according to the number of carbon atoms or alkyl groups attached on either side of the functional group. The compound with molecular formula; C4H10O, C5H10O exhibits metamerism isomerism.
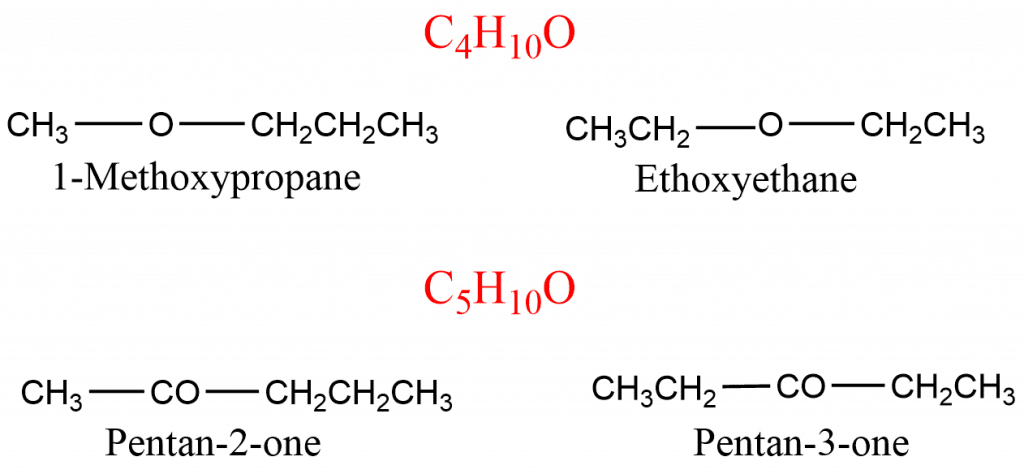
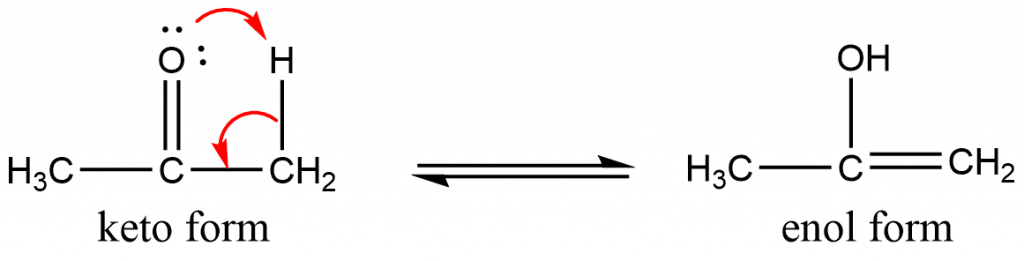
Tautomerism
Tautomerism is a phenomenon in which a single chemical molecule exists in two or more interconvertible forms via proton transfer. Such molecules/compounds are called tautomers. Keto-enol tautomerism is of a good example of it, and can be shown as:
Stereoisomerism
The phenomenon in which the isomers have the same molecular formula and constitution but have a different spatial arrangement of atoms is called stereoisomerism, and the compounds that exhibit such properties are stereoisomers. It is further sub-divided into Geometrical isomerism and optical isomerism.
Geometrical Isomerism
Geometrical isomers also known as cis or trans isomers / E or Z isomers are such compounds that have different spatial arrangements of atoms within a molecule. Both of these isomers exhibit different properties like boiling point, melting point, solubility, density, and so on.
If bigger groups in the priority order in double bond are on the same side, they are designated as Z (cis) isomer. If they are on the opposite side it is called E (trans) isomer. The cis and trans isomers have different physical and chemical properties.
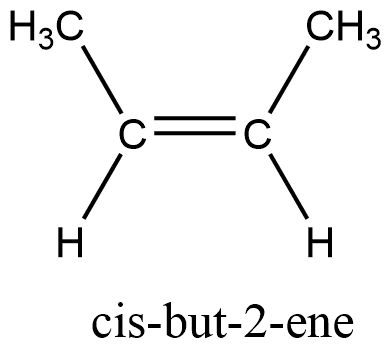
Cis-isomers
The term cis is derived from the Latin word that means ‘on the same side, and the compounds are cis isomers if the substituents are oriented in the same direction/side. Cis isomers possess dipole moments and have a lower melting point but greater solubility.
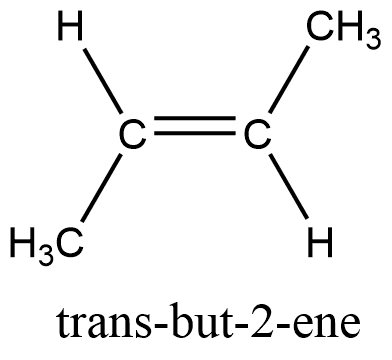
trans-isomers
The term trans isomers is also derived from the Latin word meaning on the ‘opposite side’, and the compounds are called trans isomers if the substituents are oriented on the different/opposite sides. For alkene, trans isomers have zero dipole moment and have a higher melting point.
E or Z isomerism
The word ‘E’ is derived from a German word meaning Entgegen meaning opposite, while Z is derived from the Latin word zusammen meaning together. Generally, E isomers correspond to trans isomers, while Z isomers correspond to cis isomers. However, this correlation doesn’t work for the alkenes having more than two substituents. In such a case, the higher priority groups are trans to each other in the E configuration, while such higher priority groups are cis to each other in the Z structure.
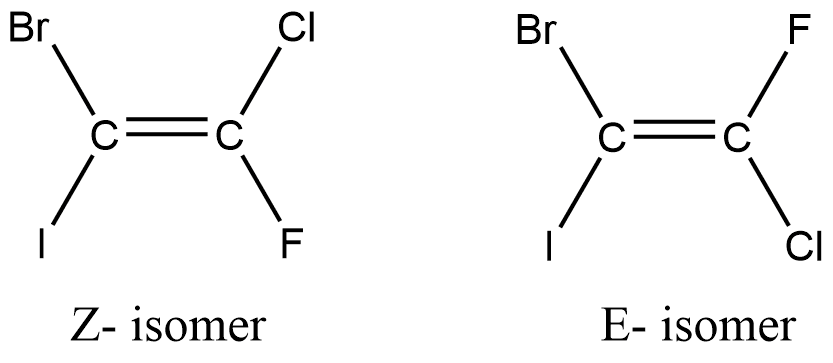
E-Z isomers are distinguished via Cahn-Ingold-Prelog priority rules.
Optical Isomerism
Optical isomers are compounds with the same chemical formula and properties that differ in how they rotate plane-polarized light, and such a phenomenon is known as optical isomerism. It is shown by the optically active compounds that rotate plane-polarized light in either clockwise or anti-clockwise direction. Such compounds are called enantiomers. The enantiomers that rotate plane-polarized light in a clockwise direction are known as (+) isomers, while those which rotate in opposite directions are known as (-) isomers/enantiomers.
The physical properties of optical isomers like melting point, boiling point, density, etc. are the same. Likewise, their specific rotations are also the same, but with opposite signs.
Conditions for Optical isomerism
- Molecule should be dissymmetric.
- Presence of chiral carbon
- Non-superimposable mirror image
Isomerism Video
MCQs/FAQs
Enantiomers
Enantiomers are the optical isomers of chiral molecules that are non-superimposable mirror images of each other.
Diastereomers
The optically active compounds which are formed by the same molecular formula but not stand as objects and mirror images are called diastereomers or diastereoisomers.
Chiral carbon
Chiral carbon is the kind of carbon that has four different groups attached to it.
Plane of symmetry
An imaginary plane that passes through atoms and bonds in a molecule so that it divides molecules into two halves each one a mirror image of the other.
Meso compound
Meso compounds are those that have two or more chiral carbon atoms as well as a plane of symmetry. Such compounds are optically inactive compounds.
Racemic mixture
An equal mixture of a pair of enantiomers is called a racemic mixture. Such compounds are optically inactive since they have opposite rotation and of
the same value, they cancel each other. It is optically inactive.
Resolution
The process of separation of a racemic mixture into the individual pure enantiomers.
Racemisation
Racemisation is the process of converting an optically active compound (dextrorotatory or laevorotatory) into racemic modification.
Racemic modification
A mixture of equal parts of enantiomers is called a racemic modification and is optically inactive.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.






