Table of Contents
ToggleSimmons smith reaction was first of all given by Simmons and smith in 1958. Olefins are stereospecifically converted into cyclopropanes by using methylene diiodide and a zinc-copper pair. This reaction is also known as Simmons-smith cyclopropanation.
Simmons smith reaction
Iodomethyl zinc iodine is known as a Simmons-smith reagent, which can be prepared by the reaction of diidomethane and a copper-zinc couple.
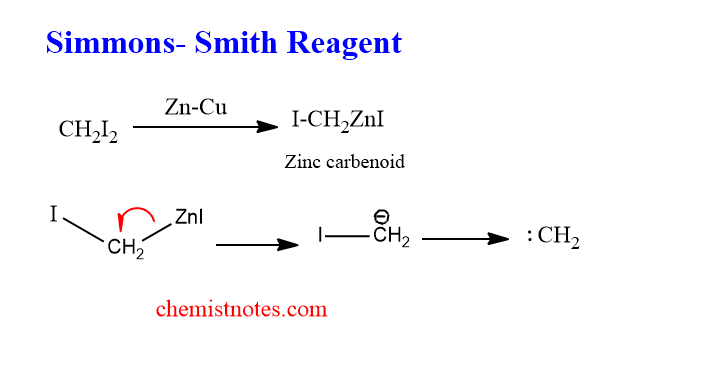
When olefins react with Simmons smith reagent, the carbene is added to the carbon-carbon double bond to form cyclopropane. This reaction is known as the Simmons-smith reaction.
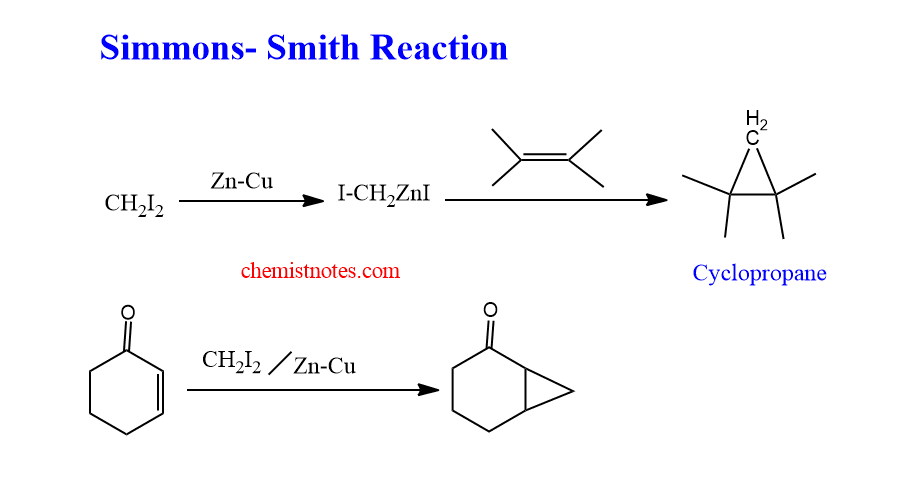
The Simmons-Smith reagent often adds to the olefins from the less hindered face during cyclopropanation. The reaction is generally efficient at rather high temperatures.
Broad substrate generality, tolerance of a range of functional groups, and stereospecificity with regard to alkene geometry are all characteristics of the Simmons-Smith reaction.
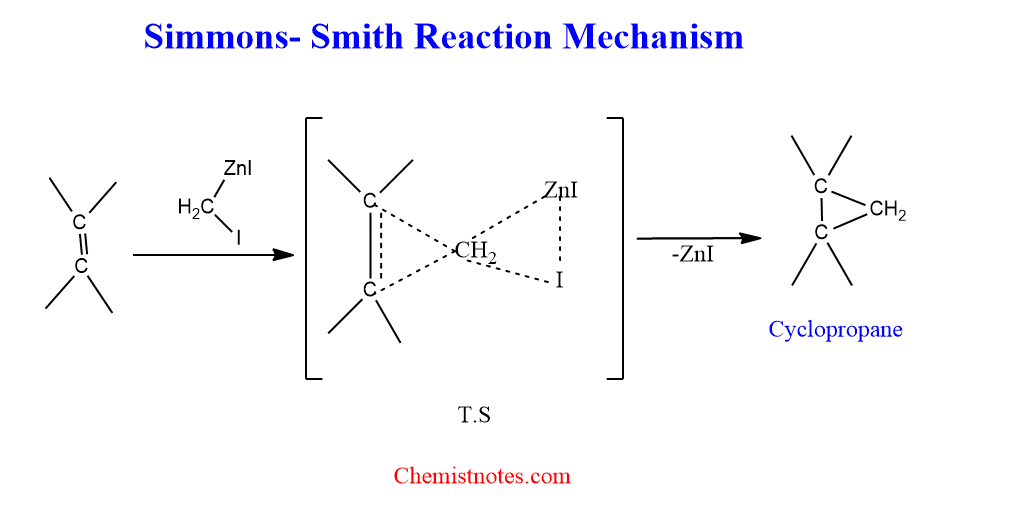
Simmons smith reaction mechanism
The methylene-to-carbon-carbon double bond addition reaction is described as a one-step process, where both new carbon-carbon bonds are formed simultaneously in a transition state.
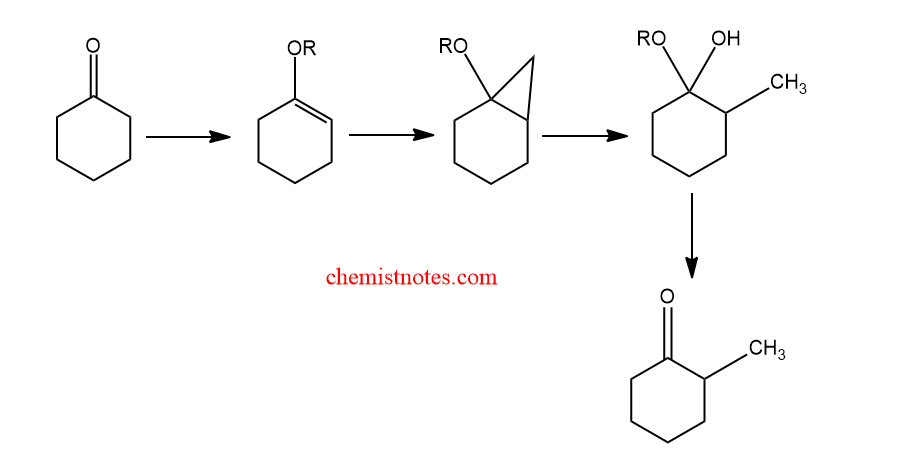
Application of simmon smith reaction
The Simmons-Smith reaction is useful for the synthesis of spirocyclic molecules.
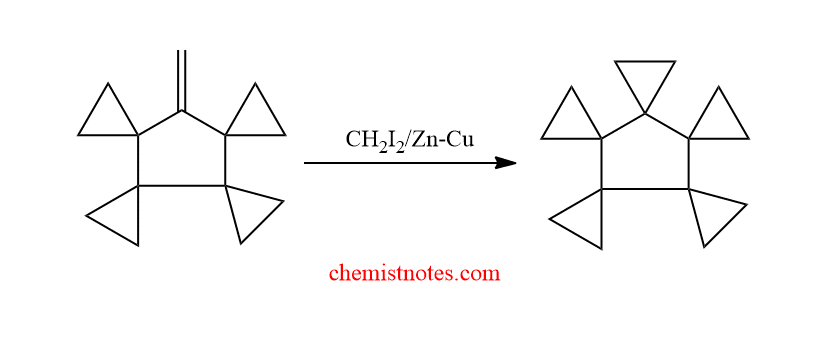
Similarly, through the use of an intermediary cyclopropane, this method has also been used to methylate ketones in the alpha position.
Simmons-smith reaction video
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro, Jr., Name Reaction and Reagents in Organic Synthesis, John Wiley & Sons, 2005
- Laue T.,Plagens A.,Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005
FAQs:
What is simmon-smith reagent?
Iodomethyl zinc iodine is called Simmon-smith reagent.






