Table of Contents
ToggleAlcohols are hydroxy derivatives of aliphatic hydrocarbons where the -OH group is bonded to a saturated carbon atom. Alcohols are represented by the general formula R-OH with the hydroxyl group being the functional group.
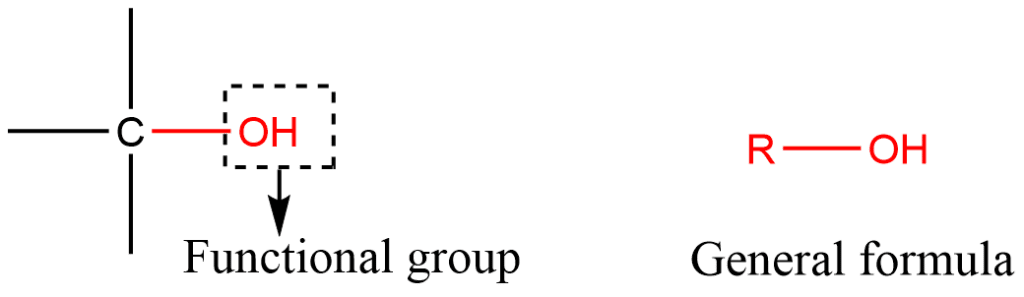
Classification of alcohols
Alcohols are classified based on the following two ways:
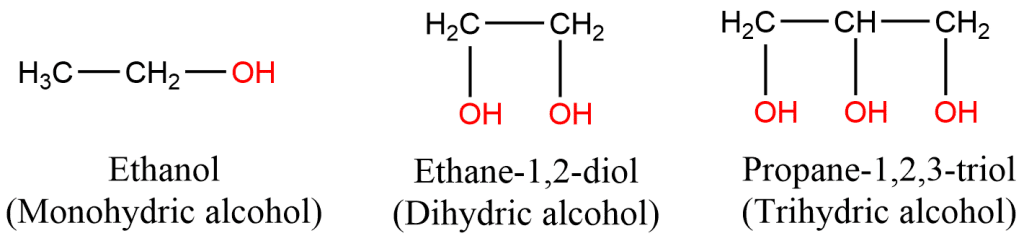
A. On the basis of a number of -OH groups: Depending on the number of -OH groups, they are called monohydric, dihydric, and trihydric.
- Monohydric alcohols: Alcohols with one hydroxyl group are called monohydric alcohol.
- Dihydric alcohols: Alcohols with two hydroxyl groups bonded on separate carbon atoms.
- Trihydric alcohols: Alcohols with three -OH groups.
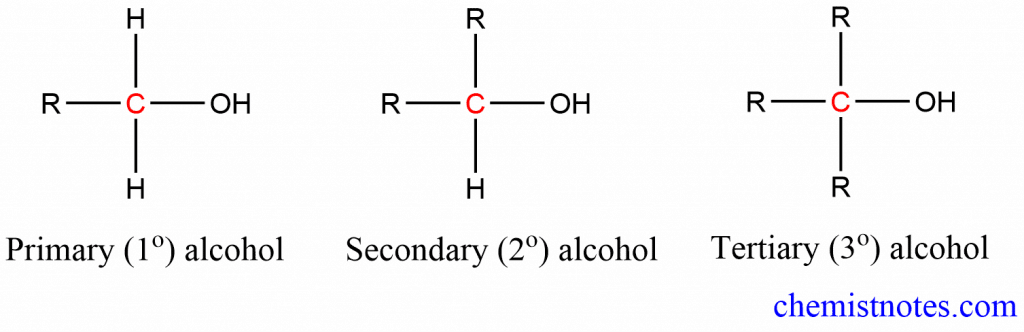
B. On the basis of carbon atom bearing -OH group: Depending on the nature of the carbon atom to which -OH group is attached, alcohols are classified into primary, secondary, and tertiary alcohol
- Primary (1o) alcohol: -OH group attached to 1o carbon atom
- Secondary (2o) alcohol: -OH group attached to secondary carbon atom i.e. carbon-containing -OH group attached to two other carbon groups.
- Tertiary (3o) alcohol: monohydric alcohols in which -OH group attached on tertiary carbon atom.
Isomerism in Alcohols
Alcohol possesses three types of structural isomerism.
Chain Isomerism: Compounds having the same molecular formula but different arrangements of carbon chains are called chain isomers and the phenomenon is called chain isomerism.

Position Isomerism: Compounds having same molecular formula but different positions of the functional groups are called position isomers. This phenomenon is called position isomerism.

Functional Isomerism: Compounds having same molecular formula but different functional groups are called functional isomers and the phenomenon is known as functional isomerism. Alcohol exhibit functional isomerism with ether.

Properties of Alcohols
Physical properties of alcohol
- Physical state: Higher members of alcohols with more than 12 carbon atoms are colorless, odorless, and waxy solids, while lower members are colorless liquids with a distinctive smell and burning flavor.
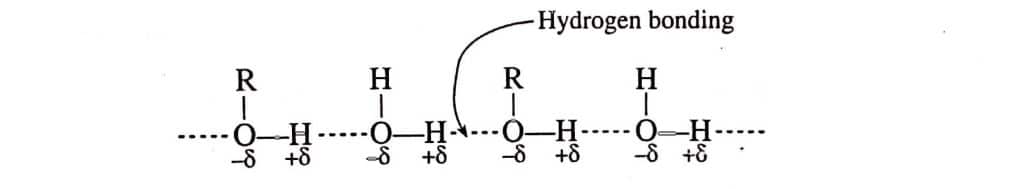
- Boiling point: The boiling point of alcohol is much higher than its isomeric ether. This is due to the presence of an intermolecular H-bond in alcohol. Similarly, The boiling points of alcohols are much higher than those of the alkanes, and alkenes of comparable molecular weights which is due to the presence of intermolecular hydrogen bonding in alcohols and its polar nature. Moreover, the boiling point rises as molecular weight increases due to increasing intermolecular forces of attraction.
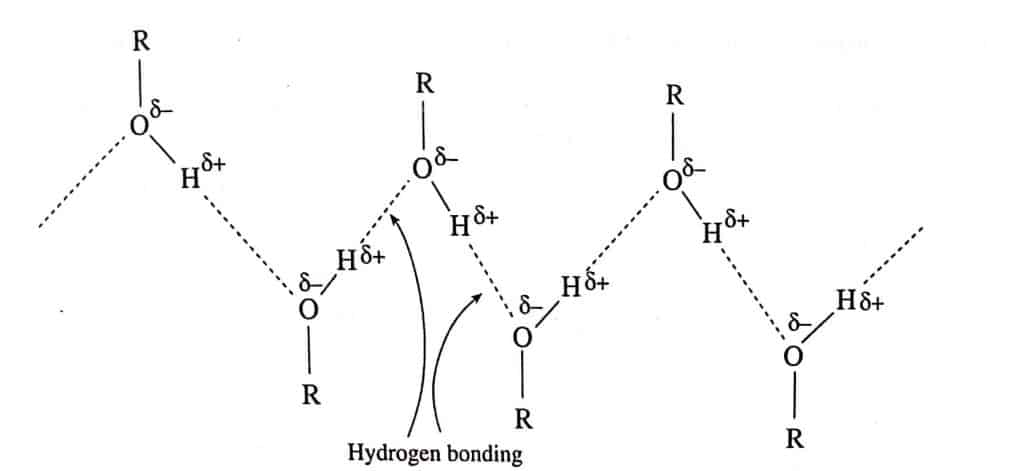
As branching increases, surface area of the molecule decreases which in turn decreases the Vander wall force of attraction between molecules, and hence the boiling point decreases. Thus, the boiling point of branched isomers is lower than that of corresponding straight-chain isomers.
- Solubility: Although the solubility of alcohols decreases with increasing molecular weight, the lowest members of the alcohol are very soluble in water. The formation of a hydrogen bond between alcohol and water molecules is what makes lower alcohols soluble in water. However, as the alcohol molecule increases in size, the alkyl group increases and hinders the formation of hydrogen bonds with water molecules. As a result, the solubility tends to decrease as the length of the carbon chain increases.
Chemical Properties of alcohols
A. Reactions involving cleavage of C-OH bond
1. Reaction with hydrogen halides: Alcohol in reaction with hydrogen halides forms alkyl halides.

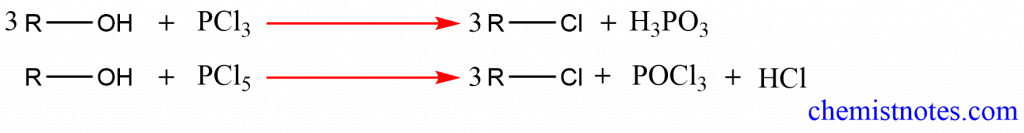
2. Reaction with phosphorus halides: When treated with phosphorus tri- or pentahalides, alcohols can be converted into alkyl halides.
B. Reactions involving cleavage of O-H bond
1. Reaction with active metals: When alcohols react with active metals like Na, K, Al, etc., the functional group of the alcohol is removed, resulting in the metal’s alkoxide. Thus alkoxides are acidic in nature.

Primary > Secondary > Tertiary is the order of acid strength. This can be explained on the basis of electron-donating nature of alkyl groups. Alkyl groups are electron-donating groups that increase the electron density on oxygen atom. As a result, the electrons of O-H bond cannot be withdrawn strongly towards oxygen atom and O-H bond remains strong. Therefore, when there are more alkyl groups present, there will be a smaller tendency for them to release proton (H+), and their acidic strength will be weaker. Consequently, the electron-releasing effect (+I effect) is greatest in tertiary alcohol and minimal in primary alcohol. As a result, acidic character decreases from primary to tertiary alcohol.
2. Reaction with carboxylic acids (Esterification): Alcohols react with carboxylic acids in the presence of conc. H2SO4 forming esters. This reaction is known as esterification.

3. Reaction with Grignard reagent: Alcohols react with Grignard reagent to form alkane.

C. Reactions involving both alkyl and hydroxyl group
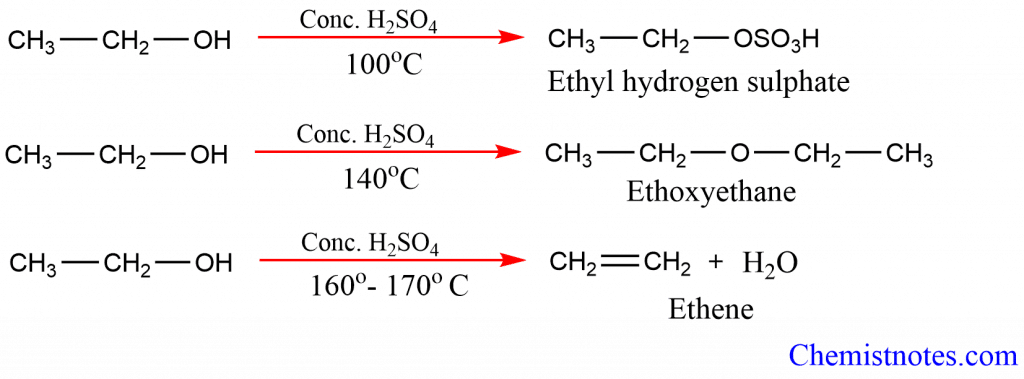
1. Dehydration of alcohol with Conc. H2SO4 : Alcohol when treated with Conc. H2SO4 undergoes dehydration forming alcohol. The order of dehydration of alcohol is tertiary > secondary > primary.
2. Dehydrogenation of alcohols: Alcohol vapors when heated with copper catalyst at 300oC, then the alcohol undergoes dehydrogenation forming different products.
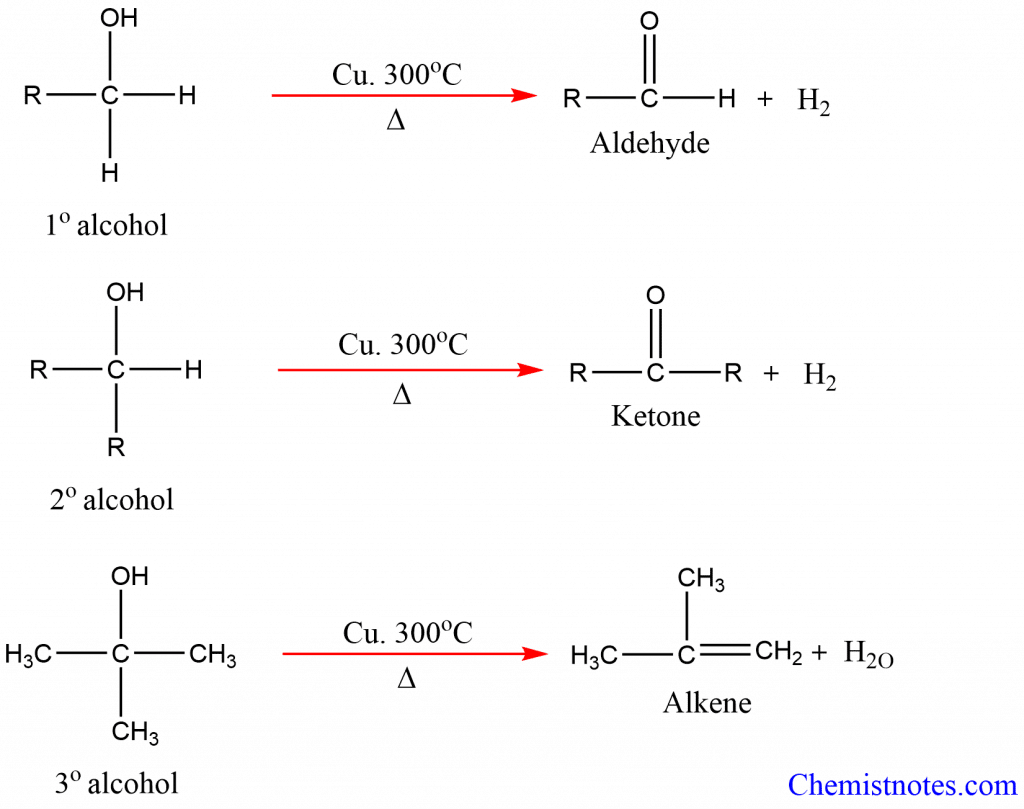
[Note: Dehydrogenation means the removal of hydrogen.]
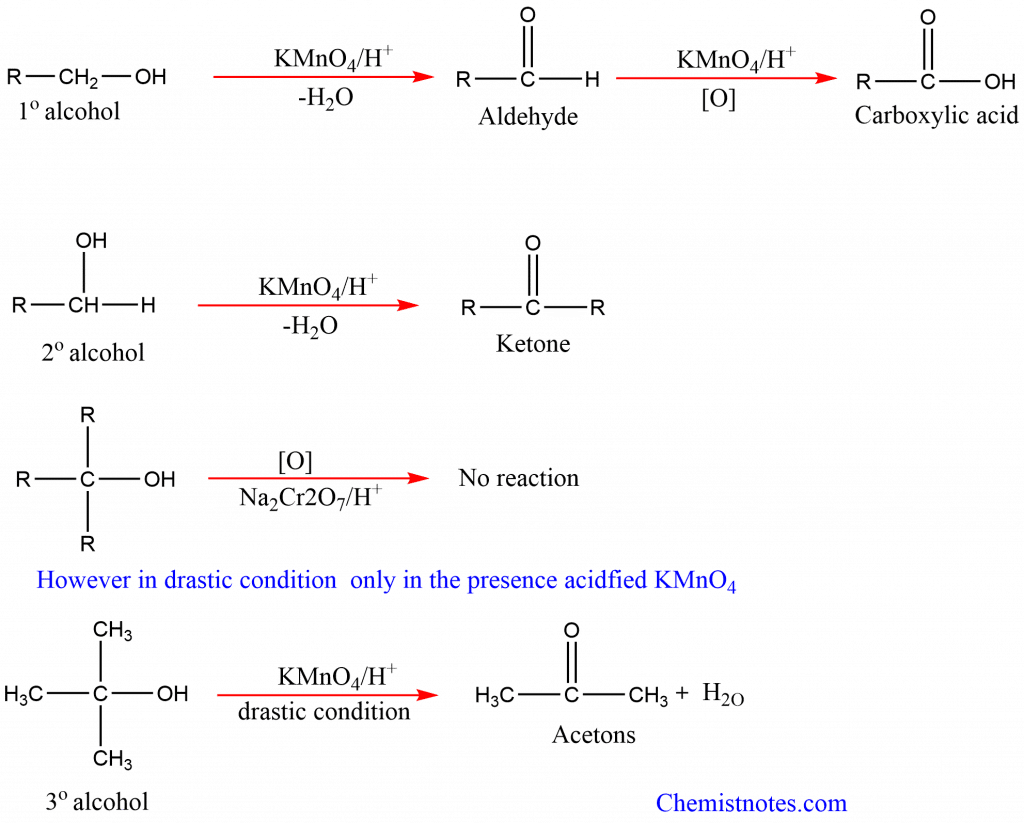
3. Oxidation of alcohols: Alcohols undergo oxidation in presence of oxidizing agents like KMnO4, K2Cr2O7, and so on. The product forms depend on the type of alcohols and nature of oxidizing agent. Primary alcohols oxidized to aldehyde which on further oxidation forms carboxylic acid. Secondary alcohols are oxidized to ketones, while tertiary alcohols don’t oxidize under normal conditions due to a lack of alpha-hydrogen. However, the reaction takes place in the drastic condition only in the presence of acidified KMnO4
Distinction of Primary, Secondary, and Tertiary Alcohol by Victor Meyer’s Method
please click on this to learn about the distinction of primary, secondary, and tertiary alcohol.
Alcohols Video
FAQS/MCQs
Alcohols are weaker acids than water, why?
In alcohols, the -OH group is connected to an alkyl group that is an electron-releasing group, while is attached to hydrogen atom water. As a result, the oxygen atom’s electron density is increased by the presence of the alkyl group in alcohol. Thus, oxygen has a reduced tendency to remove electrons from hydrogen which in turn decreases O-H bond’s polarity, making it more difficult to break. Alcohols, therefore, have weaker acidity than water.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.






