Table of Contents
ToggleIR spectroscopy involves the interaction of infrared radiation with matter and is used to determine the structure of both organic and inorganic substances.
It is also known as vibrational spectroscopy and it is based on the absorption of spectroscopy and measures the vibrations of atoms and determines the functional group. Infrared radiation in the range from about 10000 cm−1 to 100 cm−1 is absorbed and converted by an organic molecule into the energy of molecular vibration.
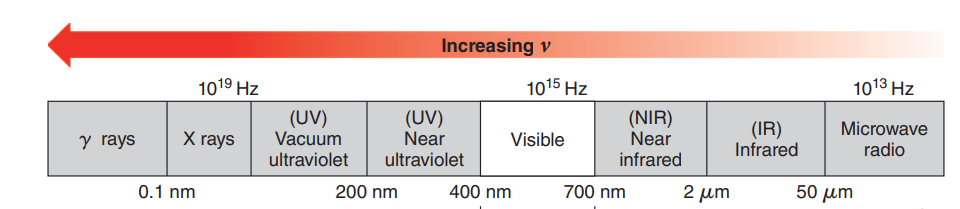
IR spectroscopy is important spectroscopy that gives sufficient information about the structure of a compound. This technique generates a spectrum containing a large number of absorption bands from which lots of information can be obtained about the structure of organic compounds. This technique can be used to identify the given compound by comparing spectra with known compounds. It is quite useful to predict the presence of functional group and these functional group absorbs IR at definite wave numbers.
Principle of IR spectroscopy
When IR radiation is absorbed by the molecules, excitation of molecules from a lower to a higher vibrational level occurs. One important point is that all bonds in a molecule are not capable of absorbing infra-red energy but only those bonds which are accompanied by a change in dipole moment will be absorbed in the infra-red region.
Since each vibrational energy level is quantized, only a definite wave number of IR light is absorbed by the molecules. The energy gap between vibrational levels is very much dependent on the nature of the bond. Therefore, each bond will absorb a characteristic frequency or wave number which allows us to determine the functional group.
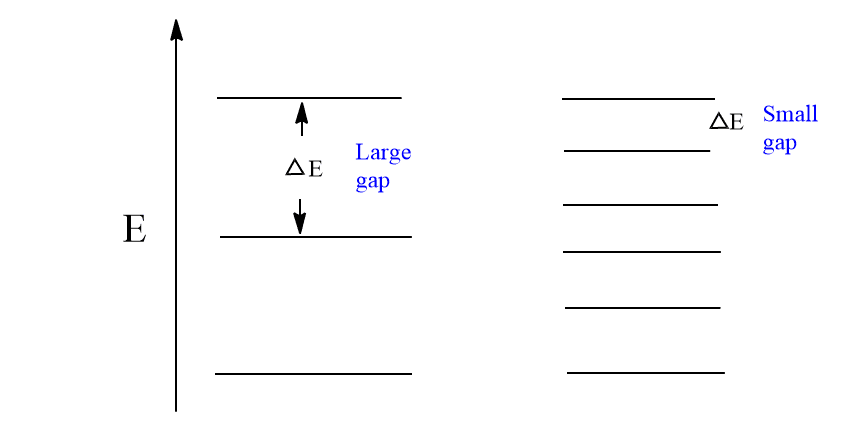
Absorption of radiation with energy equal to the difference between two vibrational energy levels (ΔE) will cause a vibrational transition to occur.
Polyatomic molecules may exhibit more than one fundamental vibrational absorption band. The number of these fundamental bands is related to the degree of freedom in a molecule. 3N-5 for linear molecules and 3N-6 for non-linear molecules.
The atom in a molecule is not held rigidly and hence can be considered as consisting of balls of different sizes tied with springs of varying lengths.

When IR light is passed through compounds, the energy is absorbed by bonds and undergoes various types of vibration.
- Stretching: In this type, the distance between the two atoms increases or decreases but the atoms remains on the same bond axis. It may symmetric or asymmetric.
- Bending: In this type, the position of the atom changes with respect to the original bond axis. It may be scissoring, wagging, and twisting.
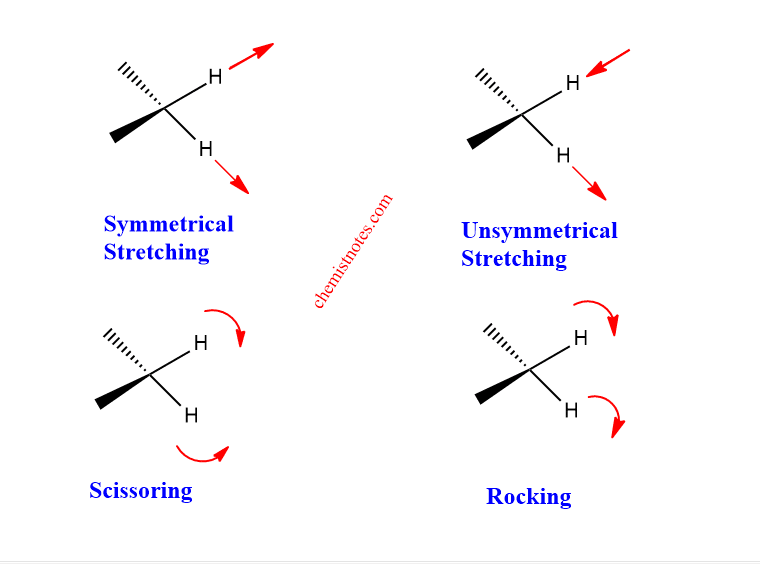
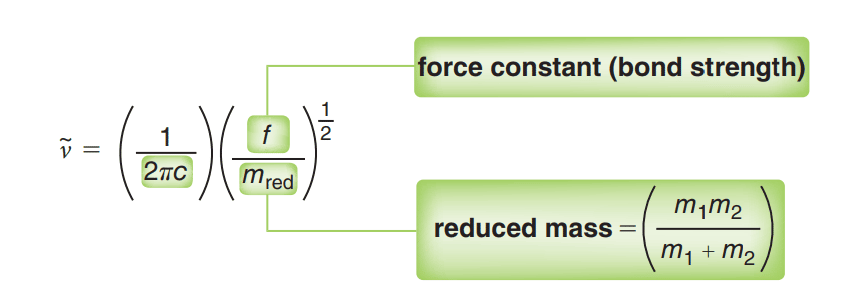
The various stretching and bending vibrations of a bond occur at a certain quantized level and the bending vibrational occurs at a lower wavenumber than the stretching vibration because the bending vibration requires lesser energy. The value of the stretching vibrational frequency or wave number can be calculated by using Hook’s law which can be expressed as:
Fingerprint and functional group region
IR spectra can be divided into two regions. One is the fingerprint and the other is a diagnostic region or functional group region.
The region ranging from 4000 cm-1 to 1300 cm-1, the high-frequency region, is called the functional group region. The characteristic of stretching frequencies for the functional group such as OH, NH, and C=O occurs in this region.
Similarly, the region of the spectrum ranging from 1300 cm-1 to 900 cm-1 is called the fingerprint region. The absorption pattern in this region is very complex. This region is very important because this region is unique for a particular chemical compound, thus helping in validation by comparison with reference compound.
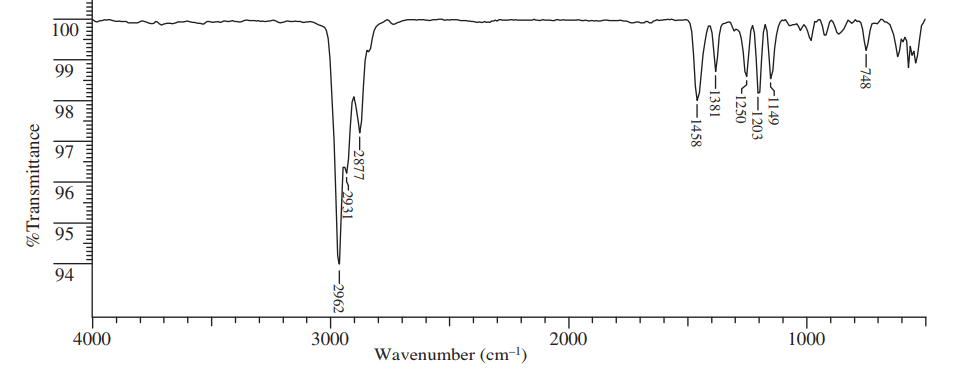
IR spectroscopy table
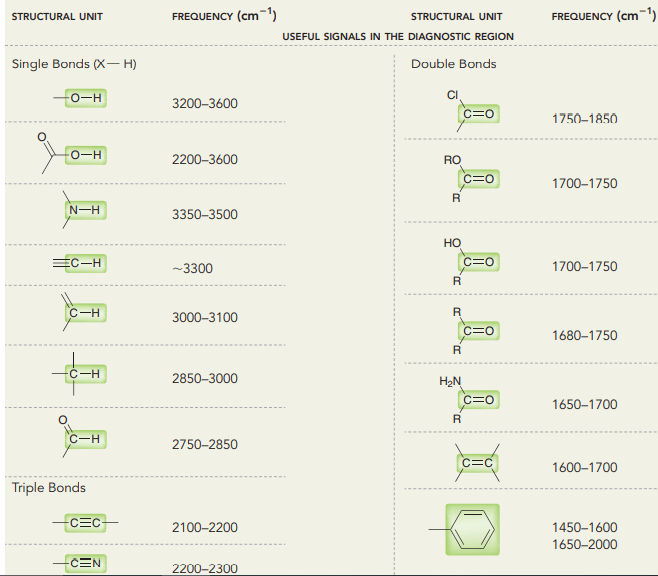
By using this table, one can easily predict which peak is due to which functional group. If you are really passionate to know about the idea of IR spectra reading, then you can read our other blog where we have given some ideas or tricks to read the IR spectra.
Application of Infrared spectroscopy
Infrared spectroscopy can be used in both qualitative and quantitative analysis.
Qualitative analysis
- IR spectroscopy measures the vibrations of atoms and determines the functional groups since every functional group absorbs IR radiation in a particular range.
- It is used in the identification of the structure of organic compounds such as Carboxylic acids, amines, alkanes, alkenes, aromatic compounds, etc. By studying the functional group region and fingerprint region, the exact structure of the compounds can be determined.
- It is used in determining the purity of the sample. The sharp and well-defined peak will be obtained for pure compounds while diffused and blurred lines spectra are formed for impure compounds.
- It is also used for studying the progress of a chemical reaction by observing the peak of the reactant and product.
- It is used to study the progress of chromatographic separations.
Quantitative analysis
- It can be used to find the concentration of toxic gases in the samples.
- It is also used to find the concentration of various compounds in different food products.
- It can be used in determining the molecular weight of polymers.
Infrared spectroscopy video
References:
- Y. R. Sharma, Elementary Organic Spectroscopy, S. Chand and Company, New
Delhi, 2013. - R. M. Silverstein, G. C. Basslerand T. C. Morrill; Spectroscopic Identification of Organic Compounds, John Wiley and Sons Inc, 1991.
What is fingerprint region?
The region of the spectrum ranging from 1300 cm-1 to 900 cm-1 is called the fingerprint region.
What is a functional group region?
The region of the IR spectrum ranging from 4000 cm-1 to 1300 cm-1 is called the functional group region.






