Table of Contents
ToggleWhat are 5-membered heterocyclic compounds?
Pyrrole, Furan, and Thiophene are the simplest 5-atom, six-π-electron heterocyclic compounds, each of which contains a single heteroatom.
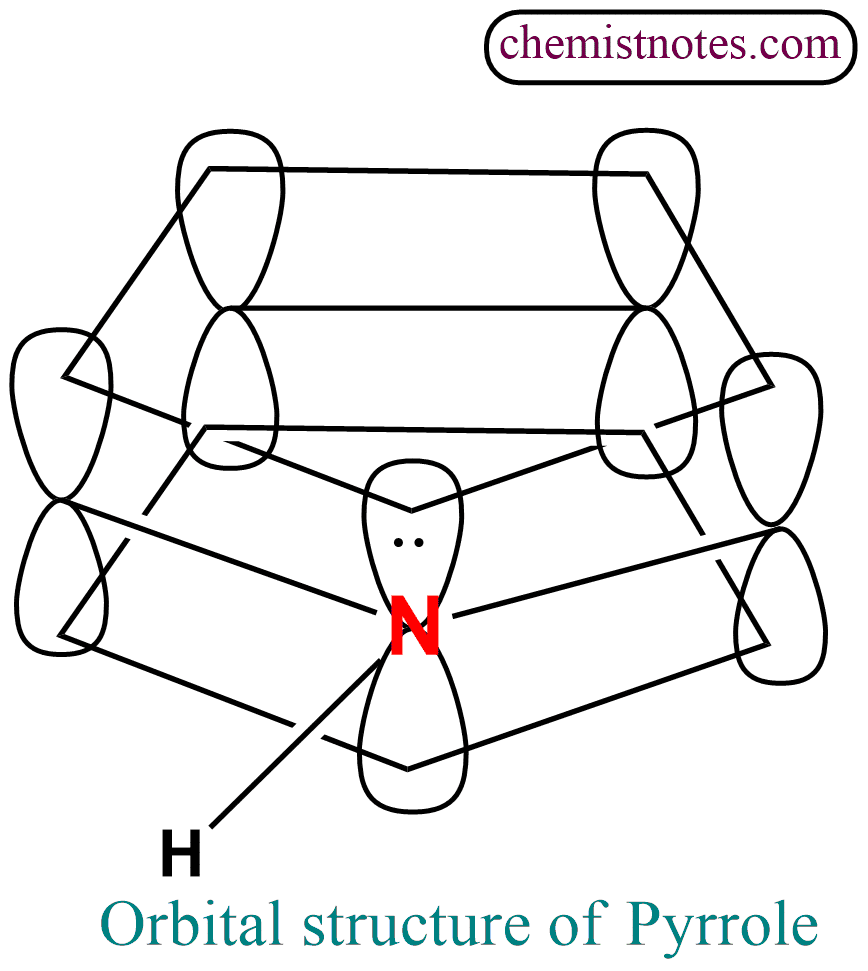
Orbital structure of pyrrole
The orbitals of carbon and nitrogen in this compound are sp2 hybridized which lie in a plane and are 120o apart. The four carbons and a nitrogen atom form a planar pentagonal ring. Each atom of the ring is held by a σ bond to three other atoms. After contributing one electron to each σ bond, each carbon atom of the ring has left 1 electron while nitrogen has left 2 electrons; these electrons occupy p-orbitals. Overlap of the p orbitals gives rise to π clouds, one above and one below the plane of the ring; the π cloud contains a total of six electrons, the aromatic sextet.
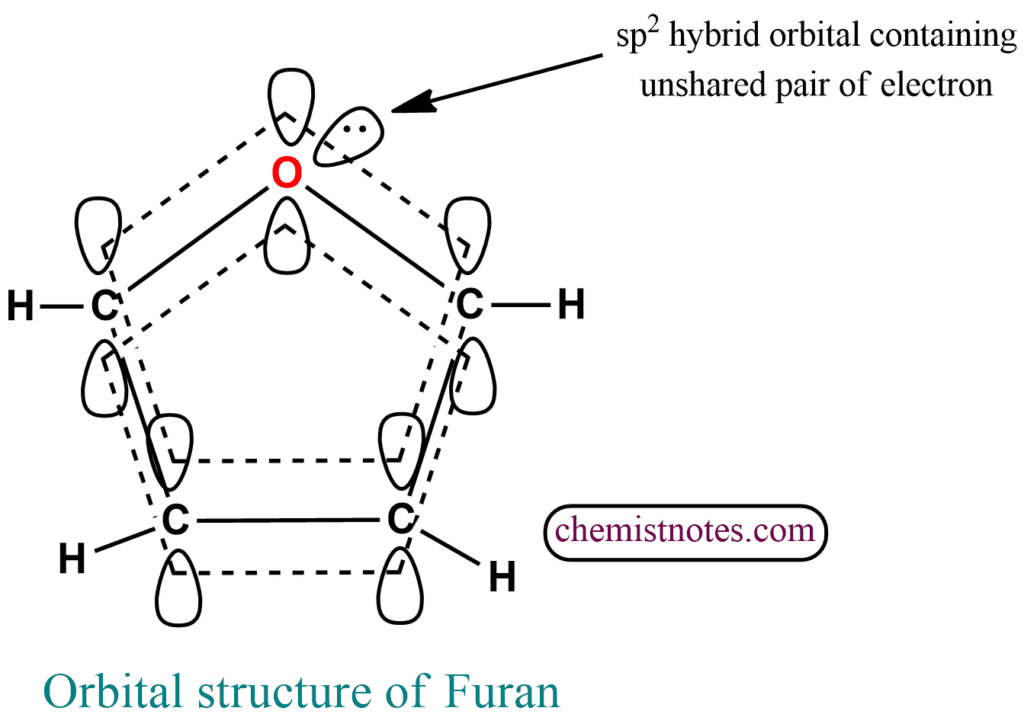
Orbital structure of furan
Furan has a planar pentagonal ring having sp2 hybridized carbon and oxygen atoms with a cyclic π electron cloud containing six electrons each lying above and below the plane of the ring. The oxygen atom in furan forms two σ bonds with two adjacent carbon atoms. Unhybridized p-orbitals having two electrons form a π bond by overlapping sideways with the p-orbital of carbon.
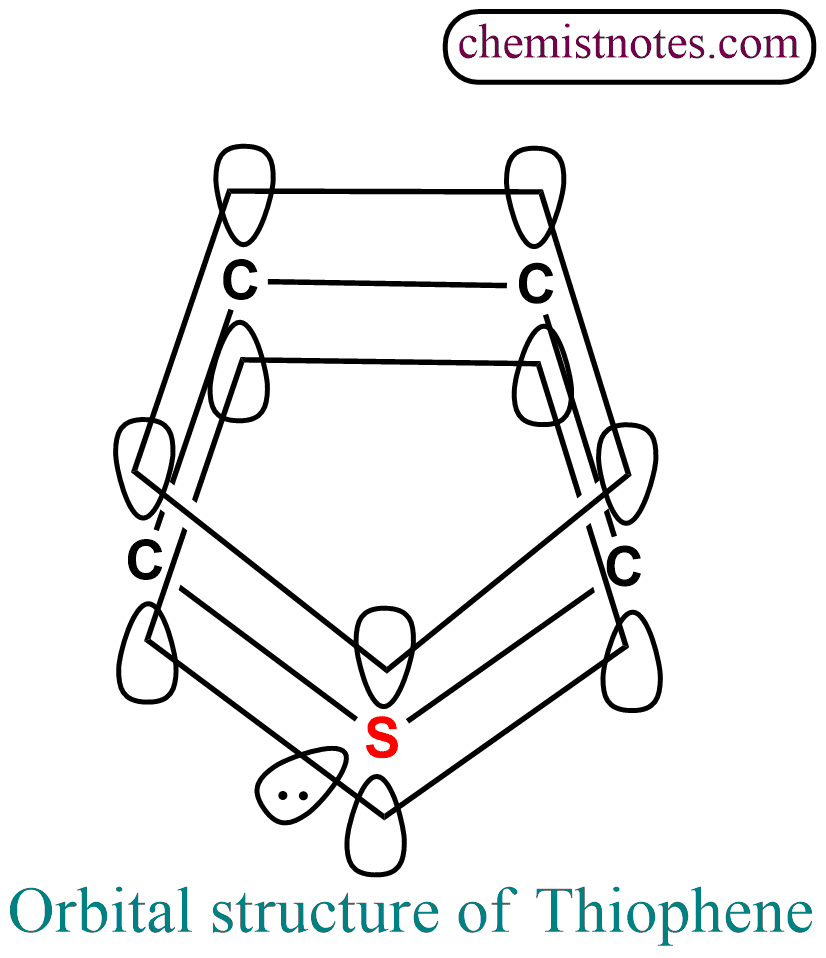
Orbital structure of Thiophene
Thiophene has a flat pentagonal ring of sp2 hybridized carbon and sulphur atoms. The structure of thiophene is similar to that of furan with the difference the oxygen has been replaced by sulphur.
Properties of Pyrrole, furan, and Thiophene
- Each of these compounds possesses the characteristics of a conjugated diene.
- These heterocycles and their derivatives most commonly undergo electrophilic substitution: nitration, sulfonation, halogenation, Friedel-Craft acylation, and even the Riemer-Tiemann reaction and coupling with diazonium salt.
- These compounds must be considered aromatic as;
- These are cyclic and planar structures.
- They undergo cyclic delocalization of π electrons.
- In these molecules, there are two carbon-carbon double bonds, i.e., 4π electrons.
- The lone pair of electrons on nitrogen, oxygen, and sulphur also joins the four π electrons to make a total of 6π electrons, so they follow Huckel’s rule (4n+2).
- They have a heat of combustion (indicating resonance stabilization) of 22–28 kcal/mol, which is somewhat less than that of the resonance energy of benzene (36 kcal/mol) but much greater than that of most conjugated dienes (about 3 kcal/mol).
Electrophilic substitution occurs in Pyrrole, furan, and Thiophene
Like other aromatic compounds, these five-membered heterocyclic atoms also undergo nitration, sulfonation, halogenation, and Friedel-Craft acylation. They are more reactive than benzene. Substitution takes place preferably at that position where the intermediate product is more stabilised. In the case of these 5-membered heterocycles, there are two positions 2 or 3 (α or β) where the substitution could take place. Let us apply this approach to the reaction of pyrrole.
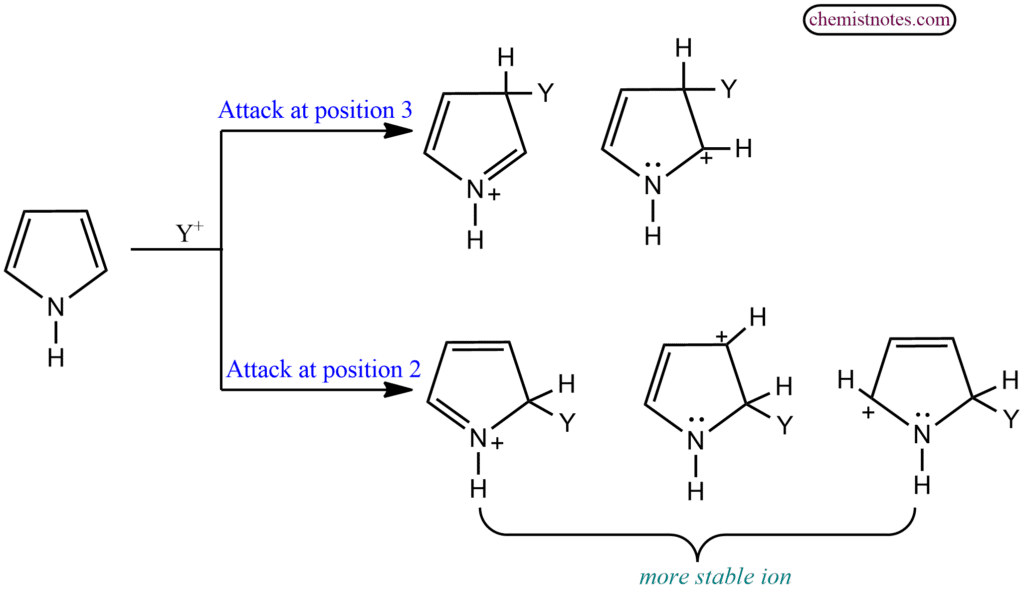
Evidently, the carbonium ion intermediate is more stabilized in case of substitution at α position. So, reactions take place predominantly at the 2-position. for example;
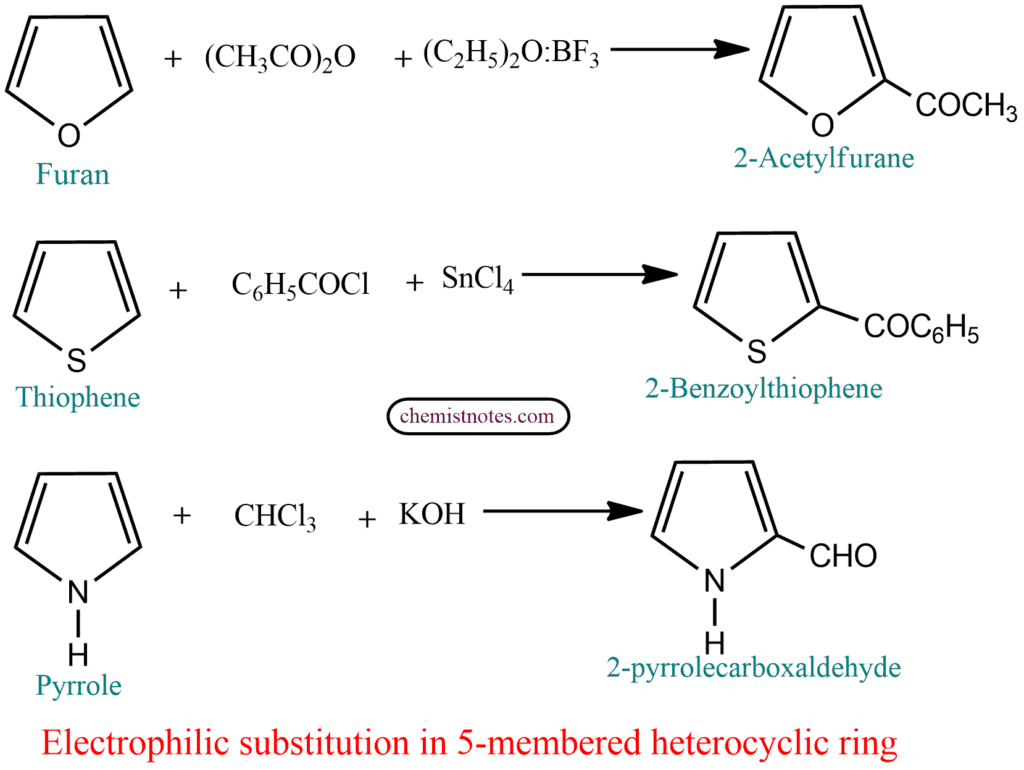
Some important facts about the Pyrrole
- Pyrrole is an extremely weak base because in pyrrole, an extra pair of electrons in nitrogen, which is responsible for the usual basicity of nitrogen compounds, is involved in the π electron cloud and is not available for sharing with acids.
- Pyrrole anion can stabilize by resonance, so it has a strong tendency to lose a proton to form a more stable anion, therefore it acts as a stronger acid.
- This is extremely reactive towards electrophilic substitution because of the high electron density in the ring is more reactive than pyridine.
Pyrrole Disorder
Pyrrole disorder is a biochemical condition in which the body produces an abnormally large number of pyrrole molecules. Symptoms commonly appear in toddlers and teens in which pyrroles have little or no use in the body. We constantly excrete pyrrole molecules (Hydroxyhemopyrrolin-2-one (HPL)) in our urine. In a healthy person, this is not a problem, but a person with pyrrole disorder can become deficient if they excrete too much pyrrole. This is because pyrroles have an affinity for zinc and vitamin B6. These pyrroles bind to zinc and B6 molecules and expel them in urine before the body can absorb them.
FAQs/MIQs
Is pyrrole aromatic?
Pyrrole is a 5-membered heterocyclic aromatic compound.
What is pyrrole disorder?
Pyrrole disorder is a biochemical condition in which the body produces an abnormally large number of pyrrole molecules.






