Table of Contents
ToggleWhat is Pyridine?
Pyridine is one of the six-membered aromatic heterocyclic compounds with the chemical formula C5H5N. It has a similar structure to benzene, in which one methine group is substituted by a nitrogen atom. It is a colorless to yellow liquid that is extremely flammable, mildly alkaline, and water-soluble, with a strong, unpleasant fish-like odor.
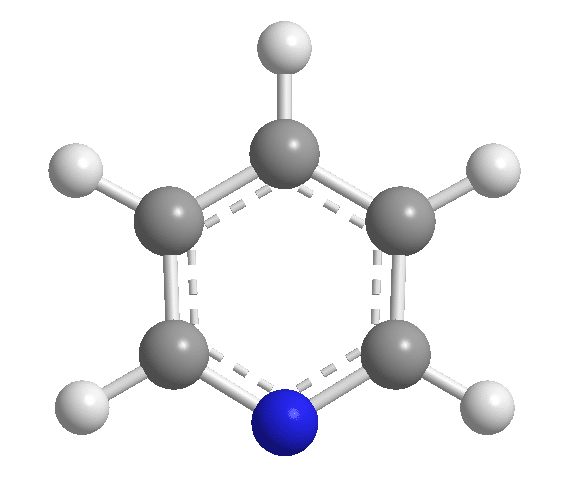
Structure of Pyridine
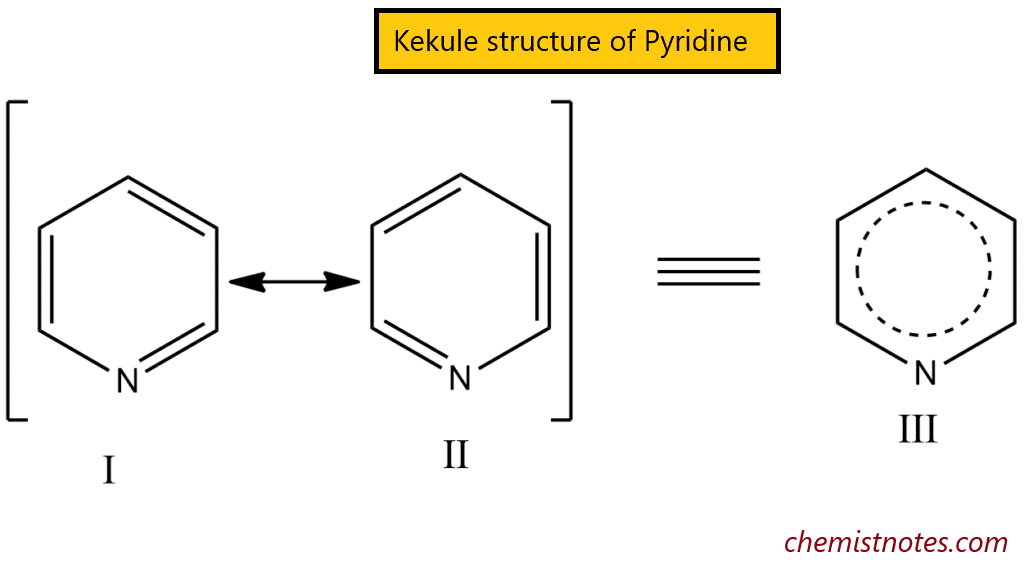
Pyridine is characterized as an aromatic compound due to its characteristics. It is flat, with bond angles of 120o, and the four carbon-carbon bonds are all the same length, as are the two carbon-nitrogen bonds. It has a resonance energy of 23 kcal/mol based on its heat of combustion. It can be described as a hybrid of Kekule structures I and II. Structure III will be applied to represent it, with the circle indicating the aromatic sextet.
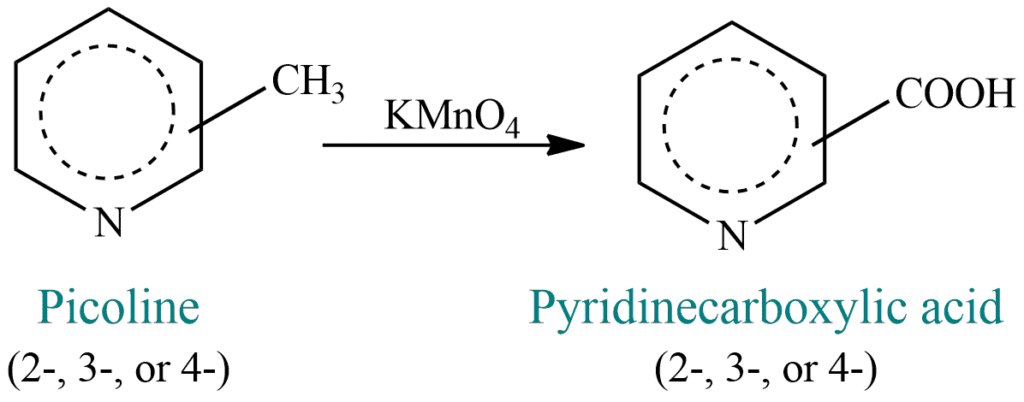
Source of pyridine compounds
It is found in coal tar. Along with it are a number of methylpyridines, the most important of which are the monomethyl compounds known as picolines. Oxidation of picolines yields pyridine carboxylic acids.
Properties of Pyridine
- Pyridine, also known as Azine, has the chemical formula C5H5N.
- The nitrogen atom in pyridine gets protonated due to its electronegative nature.
- It has a molecular weight of 79.1g/mol and a density of 982 kg/m2.
- The boiling point of pyridine is 115oC and the melting point is -41.6oC.
Basicity of Pyridine
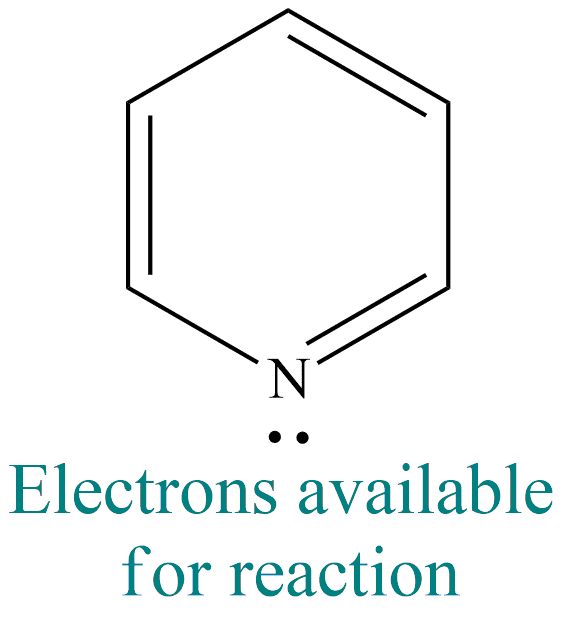
- Pyridine is a weak base, with Kb = 2.3 × 10-9. The basic nature of it is because of the unshared pair of electrons in one of the s orbitals of nitrogen.
- It is much stronger than pyrrole but much weaker than an aliphatic amine.
- Two sp2 orbitals of nitrogen overlap with the sp2 orbitals of two carbons on either side. The unhybridized orbital forms part of the delocalized electron cloud. The third sp2 orbital of nitrogen is unshared and is available for sharing with acids.
Reactions of Pyridine
The ring of azine undergoes a substitution reaction, both electrophilic and nucleophilic.
Nucleophilic Substitution in Pyridine
Due to the decrease in electron density of ring carbon atoms, they becomes susceptible to nucleophilic attack. Since positions 2- and – are very much electron deficient than position-3, nucleophilic substitution occurs readily at positions 2- and 4-, the 2-position being more preferred.
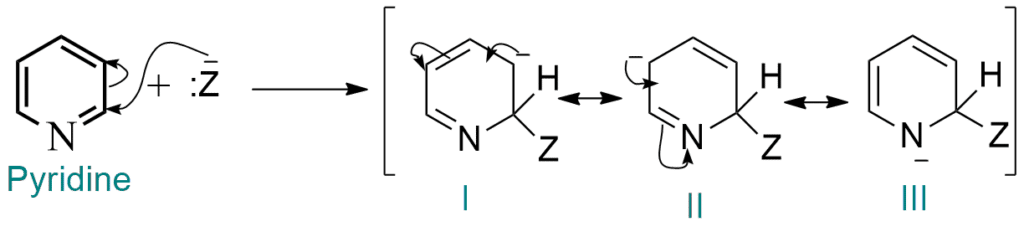
Attack at position 2 furnishes a carbanion which is a resonance hybrid of structures I, II, and III as shown above.
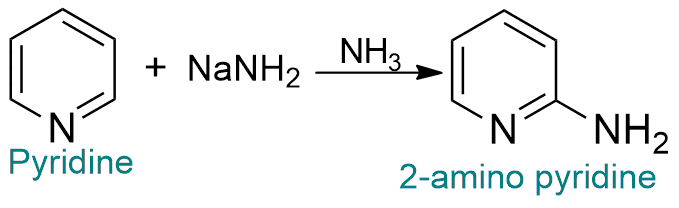
The most important example of a nucleophilic substitution reaction is the Chichibabin reaction.
Electrophilic Substitution in Pyridine
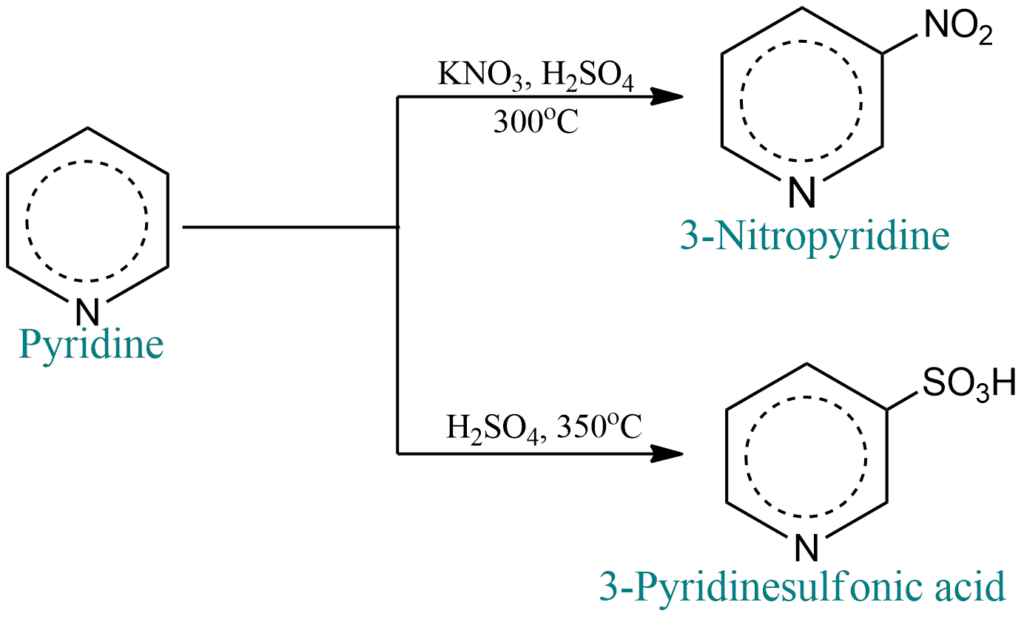
It resembles a highly deactivated benzene derivative in terms of electrophilic substitution. It only undergoes nitration, sulfonation, and halogenation at extremely high temperatures, and it does not undergo the Friedel-Crafts reaction at all.
Substitution occurs mainly at the 3- (or β-) position. For example;
Uses of Pyridine
- It is an essential raw ingredient in the chemical industry.
- It acts as a sulfonating agent.
- It works as a reducing agent.
- It’s utilized in rubber, dyes, and paints.
- It is utilized in toothpaste and toothbrushes as an antimicrobial.
- It is employed as a solvent suited for dehalogenation.
- It is employed in the pharmaceutical industry.
- It is used as a denaturant in antifreeze formulas.
- It is used as a disinfectant.
- It is used as a ligand in coordination chemistry.
FAQs
Is pyridine a base?
It is a weak base with Kb value of 2.3 × 10-9
Is pyridine aromatic?
It is characterized as an aromatic compound
What does pyridine do in a reaction?
It is used to remove the side product from the reaction.
What is Pyridine?
It is one of the six-membered aromatic heterocyclic compounds with the chemical formula C5H5N.






