Table of Contents
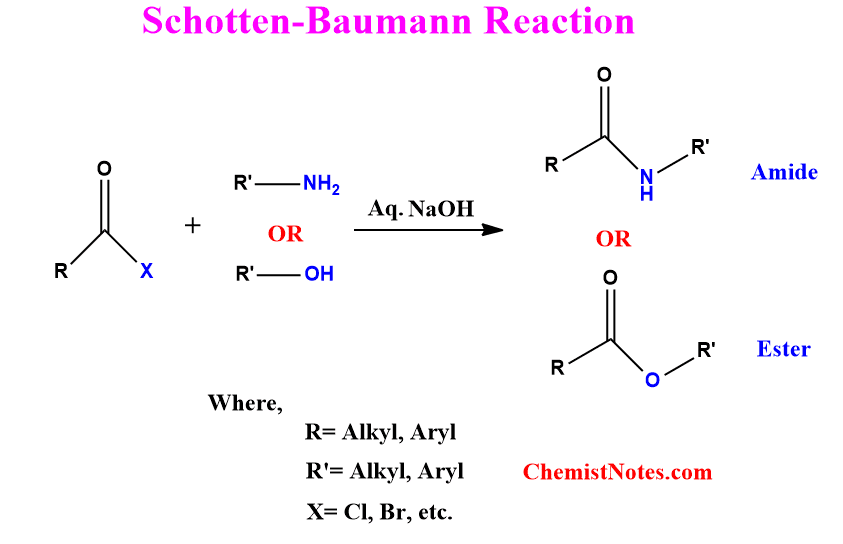
ToggleSchotten Baumann reaction was named after its initial report by Schotten in 1884 and its later extension by Baumann in 1886. This reaction is a very useful tool in organic synthesis for converting amines to amides and alcohols to esters.
Schotten Baumann reaction
Schotten Baumann reaction involves the acylation of alcohols and amines by acyl halides or anhydrides in the presence of an aqueous alkaline solution. Thus, this reaction is also known as Schotten Baumann acylation.
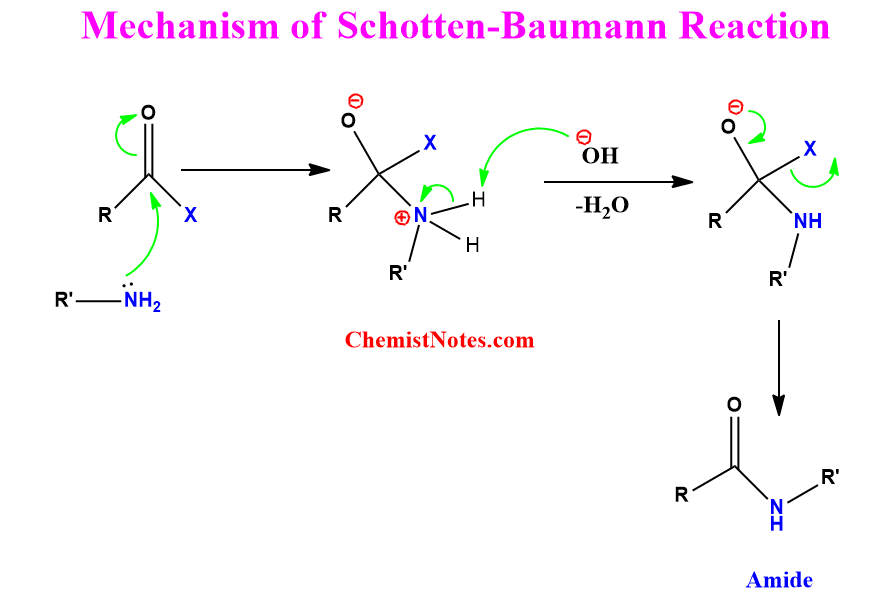
Schotten Baumann reaction mechanism
The lone pair of electrons present on the N-atom attacks the electrophilic carbonyl carbon of the acid chloride resulting in the formation of an intermediate. The OH– ion abstract proton from positively charged N-atom followed by rearrangement in which the halide ion is expelled out. Thus, the amide is formed as the final product.
What happens when alcohol is used instead of amine?
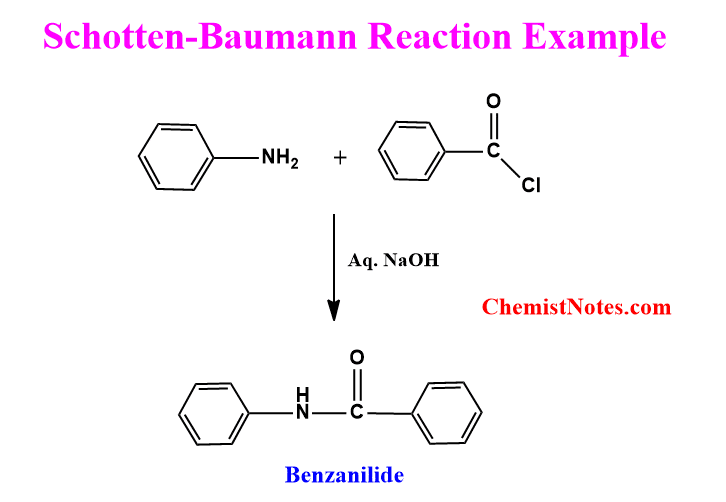
Schotten Baumann reaction example
The reaction of aniline with benzoyl chloride in the presence of aq. sodium hydroxide is an example of the Schotten-Baumann reaction. Let’s see an example.
Schotten Baumann reaction procedure
The procedure for the Schotten-Baumann reaction is described as follows:
- Take a 100 mL Erlenmeyer flask and add 2.5 mL or 2.6 g of aniline to it. Afterward, add 25 mL of 10% aq. NaOH solution.
- In a gradual and controlled manner (drop wisely), add 3.5 mL or 4.3 g of benzoyl chloride with vigorous shaking for 1 minute after each addition.
- Once the addition of benzoyl chloride is finished, tightly cork the flask and proceed to vigorously shake it for 15 minutes.
- Upon completion of the reaction, the benzoyl derivatives might precipitate as a white powder.
- Filter the white solids and perform multiple washes with water. Ultimately, recrystallize the solid by using boiling alcohol.
How will you know that the reaction is complete?
The completion of the above reaction can be predicted if there is no smell of benzoyl chloride. Moreover, the reaction mixture is alkaline when the reaction completes.
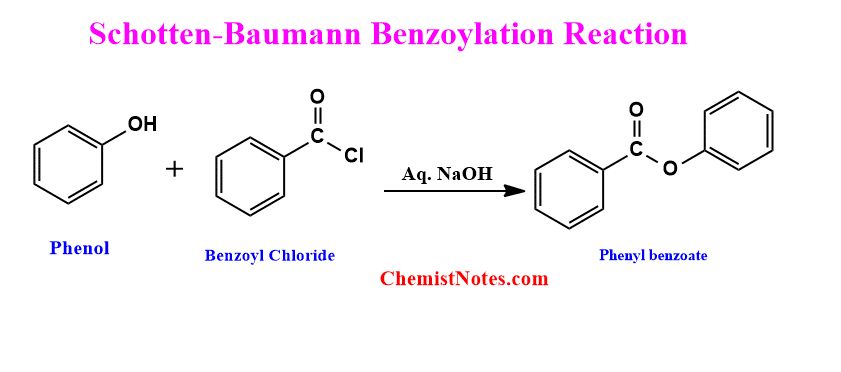
Schotten Baumann reaction of phenol
The reaction of Phenol with benzoyl chloride in the presence of aq. NaOH gives phenyl benzoate. Such a reaction is also known as the Schotten-Baumann benzoylation reaction. This is one of the popular methods for the preparation of phenyl benzoate.
Application of Schotten Baumann reaction
This reaction is applicable in organic synthesis for both laboratories as well as industrial scale. It is a widely used method in pharmaceuticals, dye synthesis, polymer chemistry, etc.
FAQs/MCQs:
What is Schotten-Baumann reaction?
Schotten-Baumann reaction is a chemical reaction used to convert amines to amides and alcohols to esters.
References:
- https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cber.188401702178






