Table of Contents
ToggleFriedel Crafts reaction is an electrophilic substitution reaction that involves the introduction of an alkyl or aryl group into an aromatic nucleus/substrate in the presence of a Lewis acid or a protonic acid as a catalyst.

Friedel Crafts alkylation reaction
Friedel crafts alkylation reaction is the alkylation of an aromatic compound by alkyl chloride in the presence of Lewis acid or protonic acid-like AlCl3 as a catalyst. The electron-donating groups facilitate the alkylation, while the electron-withdrawing groups hinder the alkylation.
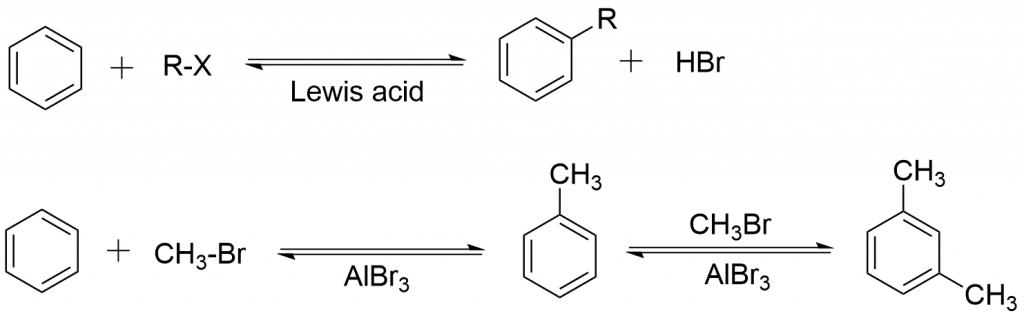
Mechanism of Friedel Crafts reaction
The reaction can be carried out in the following three steps:
Step 1: Formation of electrophile

Step 2: Electrophilic attack to the benzene ring
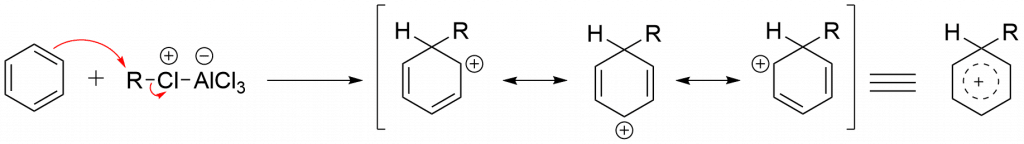
Step 3: Formation of the product via deprotonation
Applications
Friedel Crafts reaction has wide application in the preparation of substituted aromatics such as cumene and is also used to prepare fuse ring system.
Friedel Crafts acylation reaction
Friedel Crafts acylation involves the introduction of acyl group into an aromatic nucleus in the presence of Lewis acid or protonic acid-like AlCl3 as a catalyst. Because of the electrophilic character of the aromatic ring, the more enriched the electron is, the easier the reaction is; hence, electron-donating groups on aromatic rings usually favor the reaction, whereas electron-withdrawing groups usually hamper it.

Mechanism of Friedel Crafts acylation
Step 1: Formation of the donor-acceptor complex when acyl chloride interacts with AlCl3.
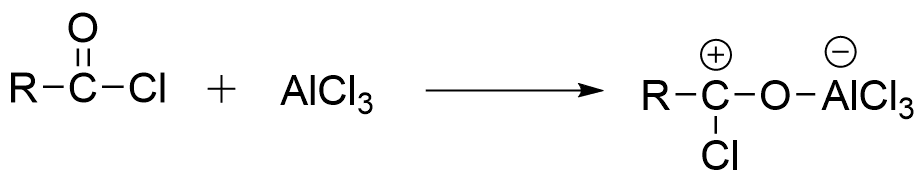
Step 2: Dissociation of complex to form alkyl or acylium ion and AlCl4 ion.
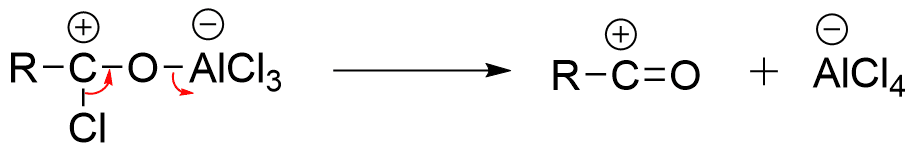
Step 3: Electrophilic attack of acylium ion on the aromatic ring.
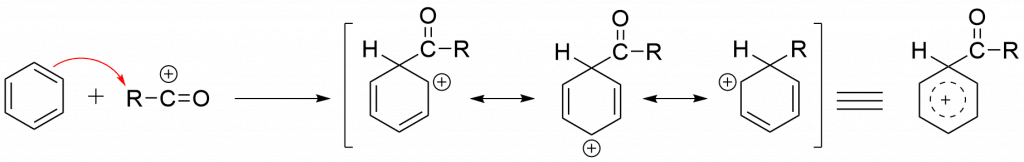
Step 4: Formation of the product via deprotonation
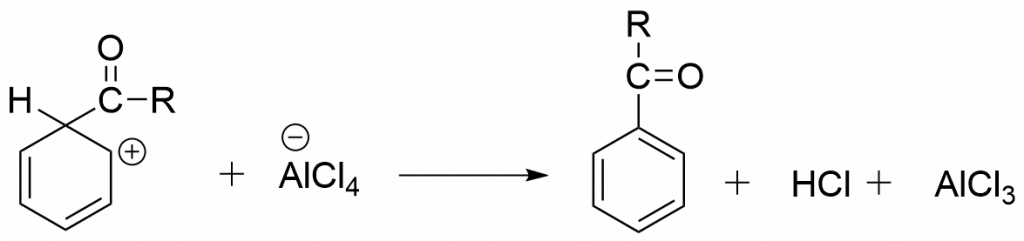
Application
Friedel Crafts acylation reaction has wide application in the preparation of aromatic derivatives such as m-nitrobenzophenone and other cyclic ketones.
FAQs/MCQs
Why aniline does not undergo Friedel crafts acylation reaction
Aniline bonds to AlCl3 and produces a coordination complex when the Friedel Crafts reaction is carried out. Because the positive charge on Nitrogen is electron-withdrawing, it pulls the electron density of the ring for future acylation processes, causing the complex to precipitate out of the acylation mixture, and hence, the acylation reaction does not occur.
Significance of Friedel Crafts reaction
Friedel Crafts reaction has wide application in the preparation of aromatic derivatives.
Does phenol undergo Friedel crafts reaction?
Phenol does not undergo Friedel Crafts reaction due to o-acylation.
Friedel Crafts reaction video
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- Lee, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2007, 129, 15438−15439.
- Silvanus, A. C.; Heffernan, S. J.; Liptrot, D. J.; Kociok-Kohn, G.; Andrews, B. I.; Carbery,D. R. Org. Lett. 2009, 11, 1175−1178.
- Pearson, D. E.; Buehler, C. A. Synthesis 1972, 533−542. (Review).
- Bourderioux, A.; Routier, S.; Beneteau, V.; Merour, J.-Y. Tetrahedron 2007, 63,9465−9475.






