Table of Contents
ToggleStrecker synthesis involves the formation of alpha-amino acids from aldehydes or ketones. This reaction generally forms a racemic mixture of alpha-amino acids. Laurent and Gerhardt were the first to report this reaction in 1838, and later, Strecker reported it in 1850. This reaction is thus known as the Strecker amino acid synthesis, Strecker condensation, or Strecker reaction.
Do you know? This reaction was the first multicomponent reaction (MCR) in organic chemistry.
Strecker synthesis
Strecker synthesis is one of the most direct and efficient methods of alpha-amino acid synthesis. When aldehyde or ketone reacts with ammonia or amines in the presence of sodium cyanide, they form alpha-amino nitriles, which can be further hydrolyzed to yield alpha-amino acid.
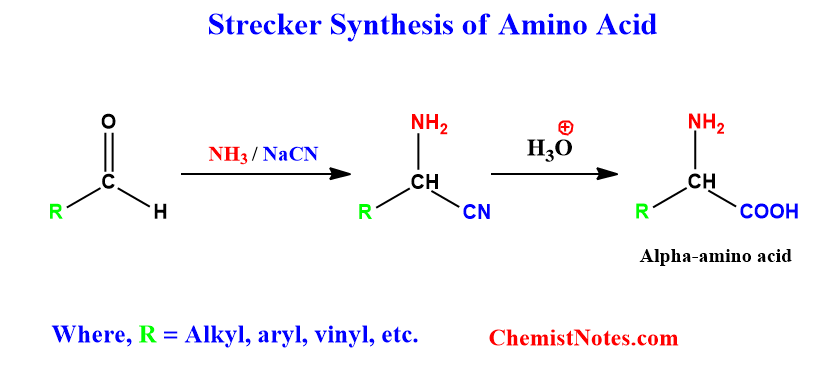
Strecker synthesis mechanism
This reaction is a two-step process. In the first step, the conversion of aldehyde into alpha-amino nitrile takes place. In the second step, the cyano group of alpha-amino nitrile is hydrolyzed to form alpha-amino acid. Let’s see the whole process mechanistically.
Step 1:
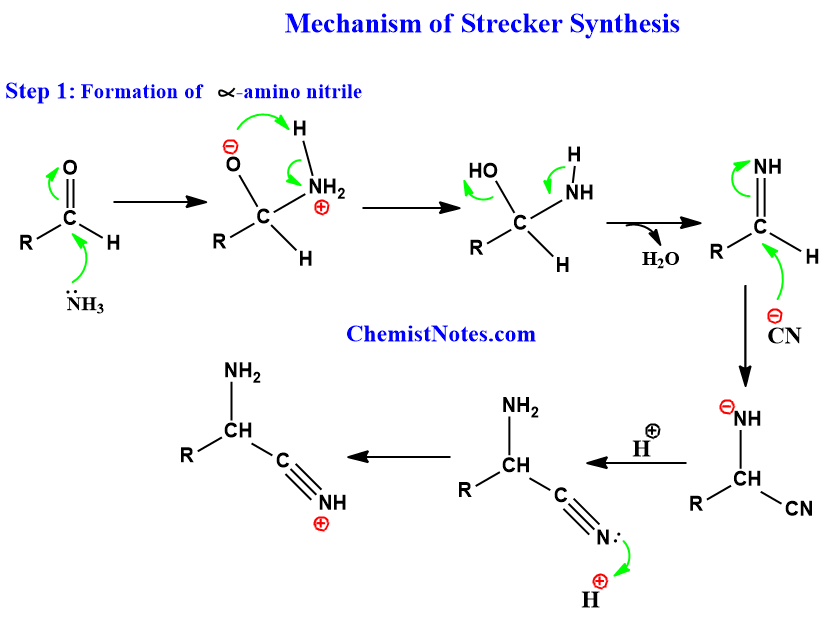
Step 2:
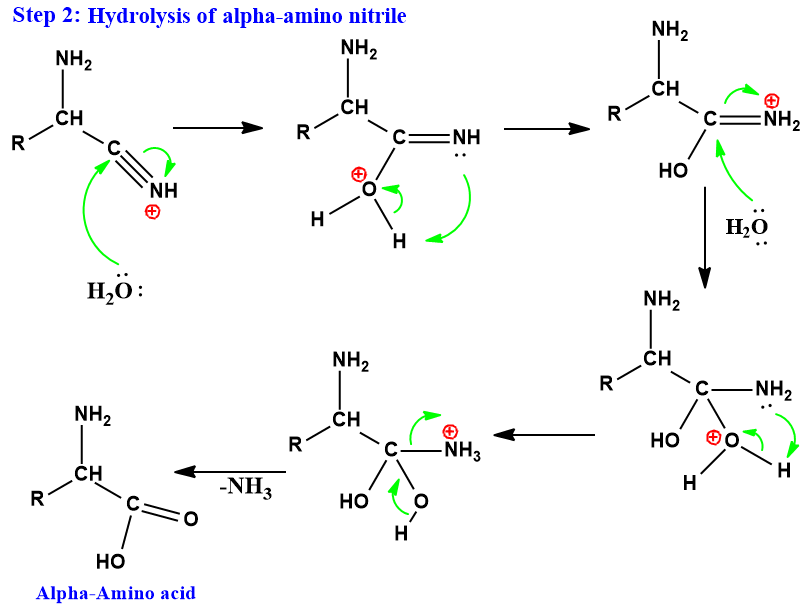
Strecker and Gabriel synthesis
Strecker and Gabriel synthesis are two different methods for the preparation of alpha-amino acids. The starting compounds and type of amino acid being synthesized determine the method of synthesis.
- In Strecker synthesis, as stated above, aldehydes or ketones are the starting materials.
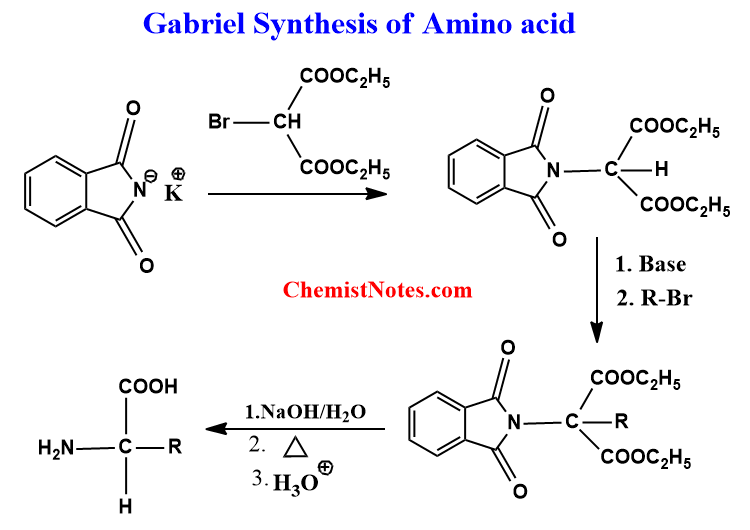
- In Gabriel synthesis, phthalimide, and alkyl halides are the starting materials. Primary amines are generally prepared by this method.
For the preparation of alpha amino acids via Gabriel synthesis, a multistep process takes place. Let’s see an example.
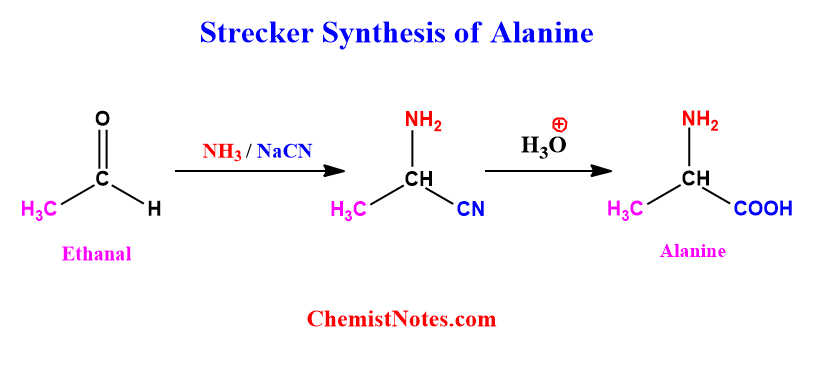
Strecker synthesis of Alanine
Alanine can be prepared via Strecker synthesis using ethanal as the starting material. The reaction involved in this process is illustrated below. The mechanism of the whole process is the same as above. Please see the above section.
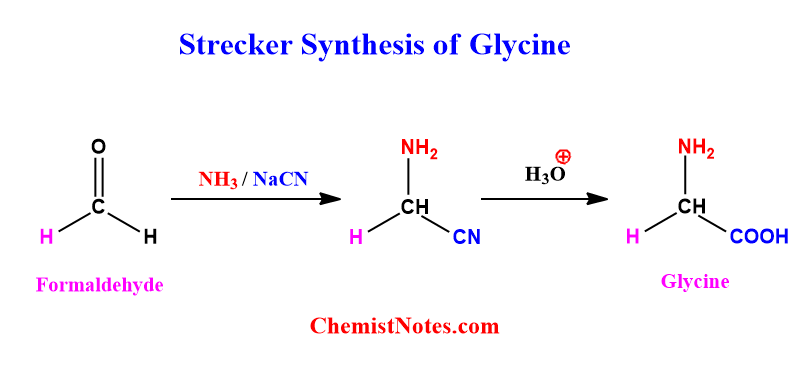
Strecker synthesis of Glycine
When formaldehyde is treated with ammonia in the presence of sodium cyanide and then followed by hydrolysis, glycine amino acid is formed. The reaction involved is shown below.
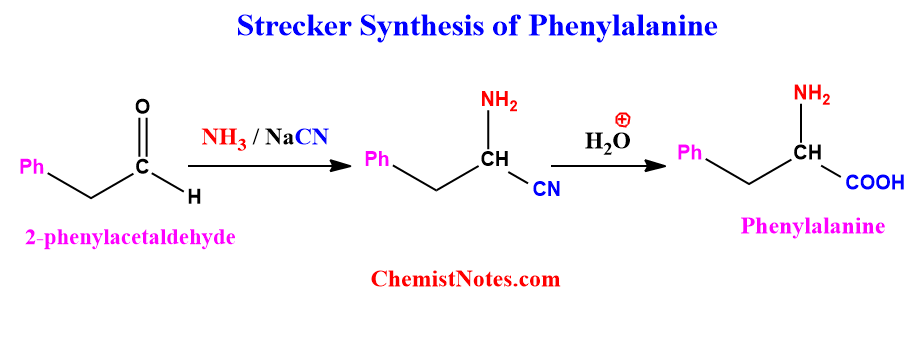
Strecker synthesis of Phenylalanine
Phenylalanine can be synthesized via the Strecker reaction using 2-phenylacetaldehyde. The reaction involved is shown below.
Application of Strecker synthesis
The Strecker synthesis is a very important reaction in organic synthesis, primarily used for the synthesis of various types of amino acids such as glycine, alanine, phenylalanine, etc.
FAQs/MCQs:
Can we make proline via Strecker synthesis?
No, we can not make proline via Strecker synthesis directly.
Why can’t you make proline via Strecker synthesis?
Proline’s structure possesses a cyclic secondary amine group. Such a structural feature is difficult to synthesize via Strecker synthesis.
Which amino acids can you make from Strecker synthesis?
Amino acids such as glycine, alanine, phenylalanine, etc. can be made from this synthesis.
What starting materials would make alanine from Strecker synthesis?
Ethanal or acetaldehyde as a starting material would make alanine from Strecker synthesis.
Strecker Reaction video
References:
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010






