Table of Contents
ToggleMagnetic equivalent protons plays a vital role in spectrum analysis and is often confused with the term chemical equivalence. It is often misinterpreted by considering it the same as chemical equivalence but in fact, all magnetically equivalent protons are chemically equivalent but all chemically equivalent protons are not magnetically equivalent. Let’s know what magnetic equivalent protons actually means.
Magnetic equivalent protons
Magnetic equivalent protons, also called spin coupling equivalent protons/nuclei, means the two equivalent protons/nuclei coupling equally to any other protons/nuclei in the same spin system present in the same set and designated as A2, X2, M2.
In order to know if the nuclei are magnetically equivalent, a geometrical relationship needs to be investigated which means if the two chemically equivalent nuclei show the same bond distance and bond angles relationship with the other nuclei, then the nuclei are magnetic equivalent.
It can be simply understood by the fact that the two chemically equivalent nuclei in the same spin system are supposed to be magnetically equivalent if they are oriented symmetrically with respect to one another and therefore, have the same chemical shift resulting in the single signal. Magnetic equivalence doesn’t exist unless the nuclei are chemical shift equivalent.
Criteria required for magnetic equivalence
- Determination of magnetic equivalence or non-equivalence can be done only if the pair of nuclei/ hydrogens are chemically equivalent
- Only if each hydrogen in a pair of chemically equivalent hydrogens has the same coupling constant values to any neighbouring hydrogen that links to both hydrogens, then the pair is magnetically equivalent.
Examples of magnetically equivalent protons
For example, in the case of 1,2 disubstituted ethane, the protons (Ha and Ha’) are subjected to the same chemical environment and coupled to both (Hb and Hb’) equally, which means their coupling constant values are the same, and therefore, they are considered as magnetically equivalent protons. The same phenomenon goes to 1,3-cyclopentadiene resulting in the magnetically equivalent protons.
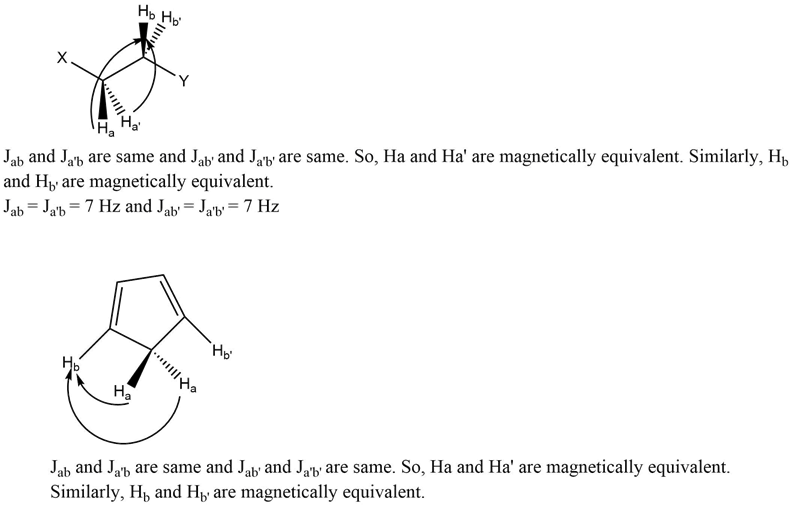
Magnetically non equivalent protons
The chemically equivalent protons are said to be magnetically non- equivalent if the two equivalent protons couple differently with the same neighbouring protons. The number, type and distribution of the substituents determine how frequently magnetic nonequivalence occurs in aromatic rings.
This can simply be demonstrated by taking an example of p-chloronitrobenzene, in which the protons ortho to the nitro group and the protons ortho to the chlorine atom are chemically equivalent protons but since, the chemically equivalent protons let’s say the protons ortho to the nitro group are magnetically nonequivalent protons as they protons couple differently to both the protons which are ortho to the chlorine. HX’ proton is ortho to HA’ but para to the HA .
Similarly, HX is ortho to the HA but para to the HA’. Hence, although, JAX and JA’X’ are the same, around 7-10 Hz and JA’X and JAX’ are also same but the value is only around 1 Hz demonstrating that HA and HA’ links differently to another same neighbouring proton. Hence, they are non-magnetically equivalent protons.
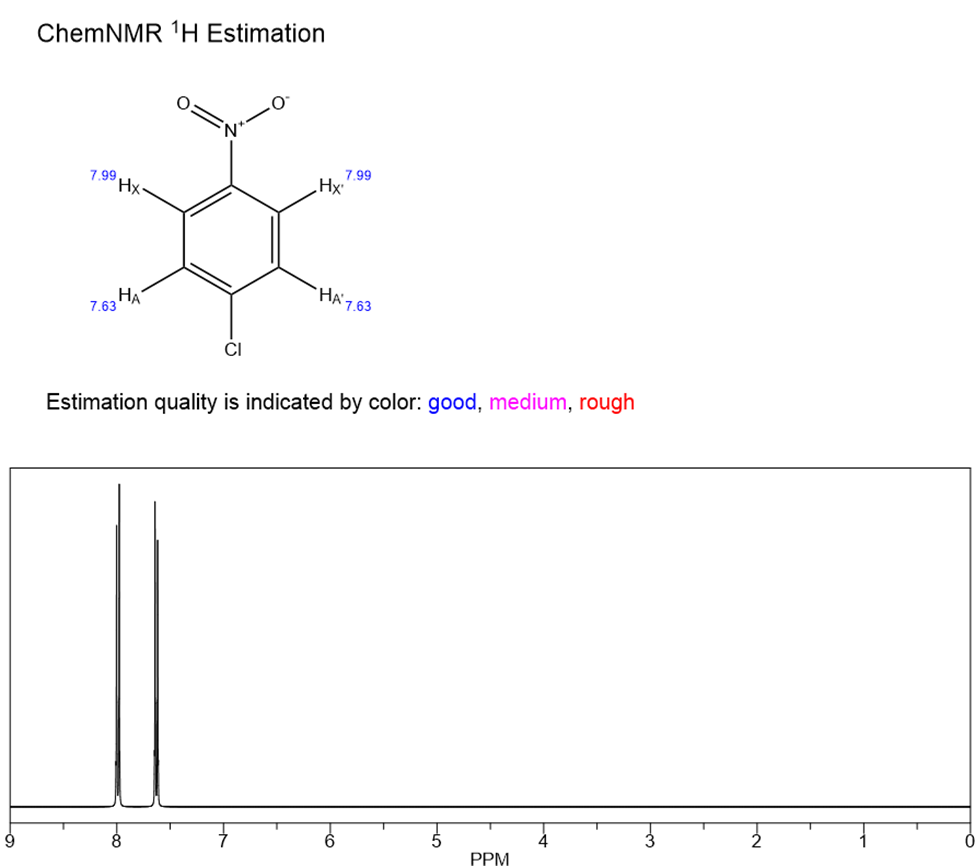
Fig: p-chloronitrobenzene in CDCl3 at 300 MHz
The spectrum is further broadened and therefore, multiplets can be easily seen.
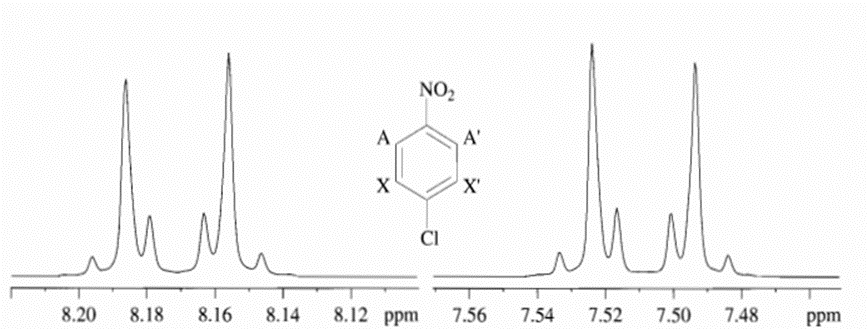
Fig: p-chloronitrobenzene in CDCl3 at 300 MHz
The main difference between magnetically equivalent and magnetically nonequivalent can be observed through spectrum as only single signal arises in case of magnetically equivalent protons which is demonstrated below as in the case of dinitrobenzene whereas in case of magnetically nonequivalent protons, the spectrum is complex and as demonstrated in above example of p-chloronitrobenzene, two multiplets which are mirror images of one another is observed.
Fig: p-dinitrobenzene in CDCl3 at 300 MHz
Chemically equivalent and magnetically equivalent protons
The difference between chemically equivalent and magnetically equivalent protons are listed below:
| Chemically equivalent protons | Magnetically equivalent protons |
| The protons that possess same chemical environment are said to be chemically equivalent protons. | The chemically equivalent protons which couple equally to other specific neighbouring protons are said to be magnetically equivalent protons. |
| Do not split each other | The protons should have same coupling constant values to other specific protons or nuclei in the molecule |
| Possess the same chemical shifts but couple differently with the same other nuclei in the same spin system | Possess the same chemical shifts in the spectrum |
| Can be determined by: • interchange through symmetry operations, • by tagging, • by rapid exchange of structures including Keto-Enol interconversion, interconversion around a partial double bond, exchange around the single bonds of the chains and many more | Can be determined by: • Building molecular models and distinguishing one atom in one set with other atom by using tags or markers. • Analyzing the bond distance and bond angles from each nucleus to the probe nucleus • If dµ/J >~8, the first order spectra is observed and the chances of protons being magnetically equivalent is high. |
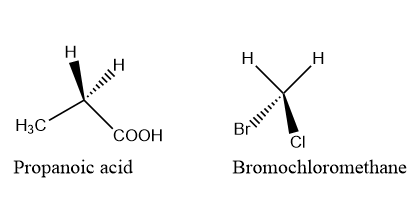
| Enantiotopic nuclei or protons are chemical shift equivalent in achiral or racemic solvent as in the example of bromochloromethane or propanoic acid. | Enantiotopic protons shows magnetic equivalence if there is a use of racemic or achiral solvents and it might not show magnetic equivalence if chiral solvent is used. |
| Diastereotopic protons may or may not be chemically equivalent protons | Diastereotopic protons will not be magnetic equivalent protons in any solvent. |
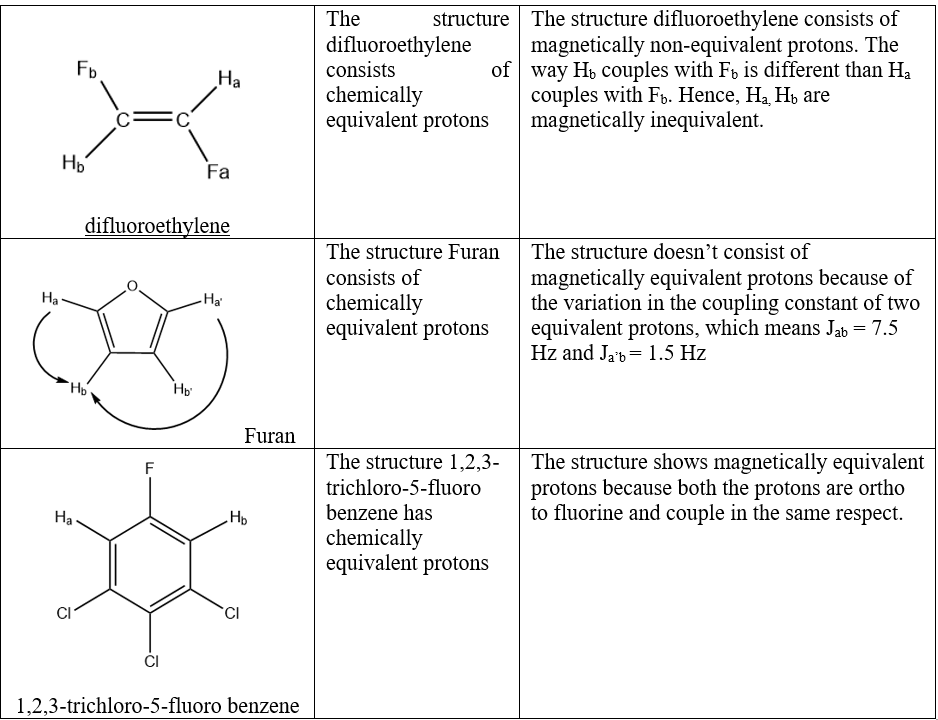
Are enantiotopic protons magnetically equivalent?
Enantiotopic protons are the protons present in achiral molecule and are the mirror images of one another if one of the protons is replaced with different group resulting in chiral molecule.
Enantiotopic protons are magnetically equivalent if achiral or racemic solvent is used. However, if chiral solvent is used, then the protons are magnetically non-equivalent because when prochiral compounds are dissolved in chiral solvents, the degeneracy of enantiotopic sites is lifted leading to spectral doubling resulting in enantiotopic discrimination and also, the splitting of NMR spectrum of racemates or other combination of optically active isomers to the subspectra corresponding to the two enantiomeric components will occur, which simply means that if chiral solvents are used then, the two enantiotopic protons will be distinguished.
Are methylene protons chemically or magnetically equivalent ?
All methylene protons are chemically equivalent but it is not necessary for it to be magnetically equivalent because for the protons to be magnetically equivalent, it must couple with the same neighbouring nuclei to the same extent.
For example in case of CH3-CH2-Y, the two methylene protons are chemically equivalent as they are subjected to same chemical environment and have same chemical shift because of free rotation around the bond but they are magnetically non-equivalent protons as HA will undergo spin-spin coupling to HB but in a different manner as HA’ couples to HB which can be understood from its Newmann projection formula :
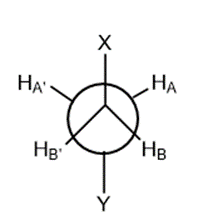
Chemically equivalent protons but magnetically nonequivalent protons
Also, in vinyl bromide methylene protons are chemically equivalent but magnetically non-equivalent.
Do magnetically equivalent protons split each other ?
Magnetically equivalent protons do not split each other and only one signal is detected in 1H-NMR spectrum as they have the same chemical shift, coupling constant, and undergoes spin-spin coupling with other nuclei at the same extent in the same spin state.
How to determine if protons are magnetically equivalent?
In order to determine if protons are magnetically equivalent or not, the bond angles and the bond distance of the chemically equivalent protons to the other specific nuclei must be considered as it must be same and the spin-spin coupling should also be at the same extent. Also, we can determine it by drawing a probe line from the probe nucleus at a right angle to the line connecting the chemically equivalent protons. If the probe line intersects the connecting line at the midpoint, then the chemically equivalent protons will also be magnetically equivalent.
For example, in case of p-chloronitrobenzene, if we draw the line connecting HA and HA’, selecting HX or HX’ as probe nucleus at a right angle connecting to HA and HA’ then the probe line will not intersect the connecting line at midpoint, demonstrating that HA and HA’ are magnetic nonequivalent protons.
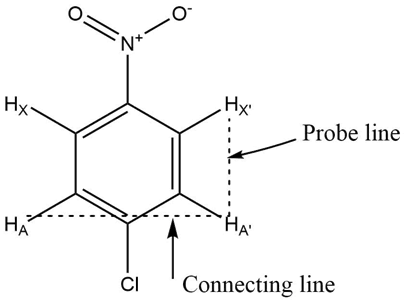
Fig: p-chloronitrobenzene
Published By: Soniya Joshi
FAQs/MCQs:
What do you mean by magnetic equivalent protons?
Magnetic equivalent protons, also called spin coupling equivalent protons, means the two chemically equivalent protons coupling equally to any other protons in the same spin system present in the same set.
References:
- Lesot, Philippe, Christie Aroulanda, Herbert Zimmermann, and Zeev Luz. “Enantiotopic Discrimination in the NMR Spectrum of Prochiral Solutes in Chiral Liquid Crystals.” Chemical Society Reviews 44, no. 8 (2015): 2330–75. https://doi.org/10.1039/C4CS00260A.
- Ault, Addison. “Test for Chemical Shift and Magnetic Equivalence in Nmr.” Journal of Chemical Education 51, no. 11 (November 1974): 729. https://doi.org/10.1021/ed051p729.
- Silverstein, Robert M., and G. Clayton Bassler. “Spectrometric Identification of Organic Compounds.” Journal of Chemical Education 39, no. 11 (November 1962): 546. https://doi.org/10.1021/ed039p546.
- Perrine, Danial M. “Synthesis of a Lactone.” Journal of Chemical Education 75, no. 7 (July 1998): 803. https://doi.org/10.1021/ed075p803.1.






