Table of Contents
ToggleHybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. The new orbitals produced in this way are known as hybrid orbitals. The shape of a covalent molecule can be predicted through hybridization.
Types of Hybridization
The important types of hybrid orbitals are listed in table below.
| Hybridization | Orbitals mixed | shape | example of molecule |
| sp | s+p | Linear | BeF2, BeCl2 |
| sp2 | s+p+p | Triangular planar | BF3, C2H4 |
| sp3 | s+p+p+p | Tetrahedral | CH4, NH3 |
| sp3d | s+p+p+p+d | Trigonal bipyramidal | PCl5, SF4 |
| sp3d2 | s+p+p+p+d+d | Octahedral | SF6, |
| sp3d3 | s+p+p+p+d+d+d | Pentagonal bipyramidal | IF7 |
| dsp2 | d+s+p+p | Square planar | [Ni(CN)4]2- |
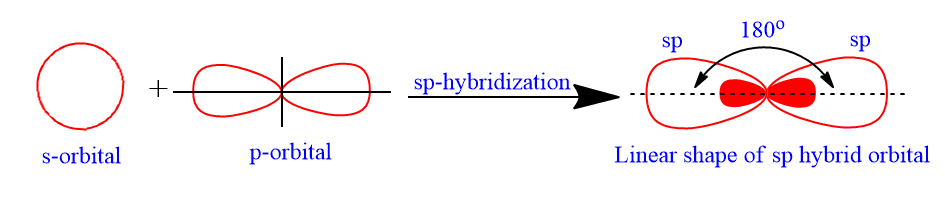
sp-hybridization (diagonal linear hybridization)
sp-hybridization is the process of one s-atomic orbital intermixing with one p-atomic orbital to form two equivalent hybrid orbitals. The two new orbital formed by this process are called sp-hybrid orbital. The two new orbital formed by this process are called sp-hybrid orbital.
Each sp-hybrid orbital has 50% s-character and 50% p-character. The hybrid orbitals are aligned in a linear fashion. As a result, sp-hybrid orbitals have a linear form with a bond angle of 180° between their axes. Hence, the covalent molecule with central atom having sp-hybridisation has linear geometry.
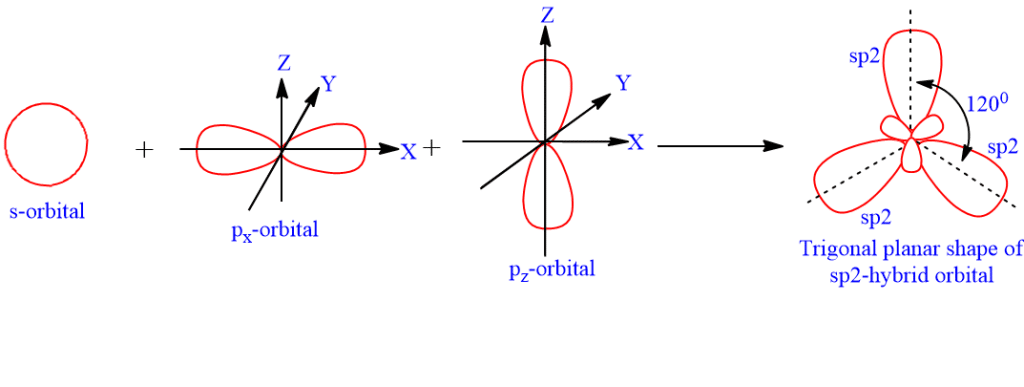
sp2-hybridization (trigonal hybridization)
sp2-hybridization is the process of combining one s orbital with two p-atomic orbitals of almost similar energy to generate equivalent hybrid orbitals. The three hybrid orbitals are called sp2 hybrid orbitals. Each sp2-hybrid orbital has 33.33% s-character and 66.66% p-character. These three sp2 hybrid orbitals are in the same plane and pointing towards the corner of an equilateral triangle. The geometry of the sp2- hybrid orbital is trigonal planar, with a bond angle of 120o between the orbital axes.
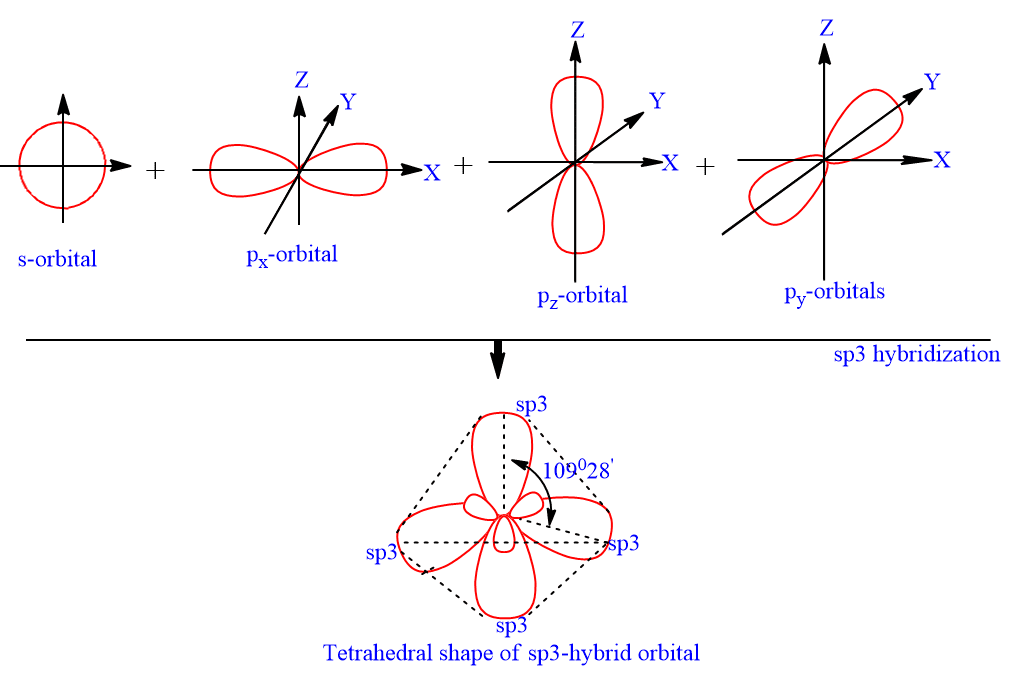
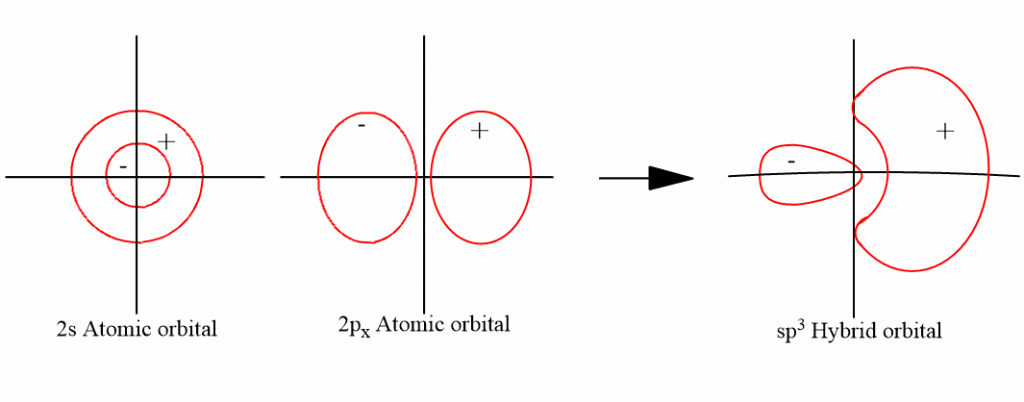
sp3-hybridization (tetrahedral hybridization)
The sp3 hybrid orbital is a blend of one s orbital and three p orbitals. Mixing one s and three p orbitals in this way gives four sp3 hybrid orbitals. Each sp3-hybrid orbital has 25% s-character and 75% p-character. The hybrid orbitals directed towards the four corner of regular tetrahedron, hence it has tetrahedral shape having bond angle 109o28‘.
| Orbital | Relative bond strength |
| s | 1.0 |
| p | 1.73 |
| sp | 1.93 |
| sp2 | 1.99 |
| sp3 | 2.00 |
d2sp3 or sp2d2 (octahedral hybridization)
When the dx2-y2 and dz2 orbitals are combined with and s orbital and set of px, py and pz orbitals, a set of equivalent orbitals with lobes directed to the vertices of an octahedron can be formed.
dsp2 (square planar hybridization)
A dx2-y2 orbital, an s orbital, and px and py orbitals can be combined to give a set of equivalent hybrid orbitals with lobes
sd3 (tetrahedral hybridization)
An s orbital and the set dxy, dyz, dzx, may be combined to give a tetrahedrally directed set of orbitals.
dsp3 or sp3d (trigonal bipyramidal hybridization)
The orbitals, s, px, py, pz, and dx2-y2 may be combined to give a nonequivalent set of five hybrid orbitals directed to the vertices of a trigonal bipyramid.
sp3d3 (pentagonal bipyramidal hybridization)
The orbitals s, p, px, py, pz and three d-orbitals may be combined to give a set of seven hybrid orbitals. Five of them points towards the vertices of a regular pentagon while others are perpendicular to plane.
Some important features of Hybridization
- The orbitals involved in hybridization must only have a slight variation in energy.
- Half filled, completely filled and vacant orbitals can take part in hybridization.
- The number of hybrid orbitals involved in hybridization is equal to the number of atomic orbitals involved in hybridization.
- The hybrid orbitals have the identical energy and shape.
- Due to their greater overlap, hybrid orbitals can form stronger bonds than pure atomic orbitals.
- The electron density of the hybrid orbital is concentrated on one side of the nucleus, with one lobe slightly bigger than the others. As a result, hybrid orbitals have a stronger bond.
- The geometry of molecule depends on the type of hybridization involved in central atom. The type of hybridization depends on the number of hybrid orbitals i.e. sum of sigma- bond pairs and lone pairs present on the valance shell of the central atom of molecule.
FAQs
What is hybridization?
Combining or mixing the wave functions for the atomic orbitals is called hybridization.
Hybridization of BF3?
The hybridization of BF3 is SP3
Hybridization of NH3?
The hybridization of NH3 is SP3.
Hybridization of XeF2?
The hybridization of XeF2 is SP3d.
Hybridization of XeF6?
The hybridization of XeF6 is SP3d3.
Hybridization of H2O?
The hybridization of H2O is SP3.
Hybridization of BeCl2?
The hybridization of BeCl2 is SP.
Hybridization of CH4?
The hybridization of CH4 is SP3.
Hybridization of NO2?
The hybridization of NO2 is SP2.
Hybridization of SO3?
The hybridization of SO3 is SP2.
Hybridization of C2H4?
The hybridization of C2H4 is SP2.
Hybridization of XeOF4?
The hybridization of XeOF4 is sp3d2 or d2sp3.
Hybridization of CH3?
The hybridization of CH3 is SP2.






