Table of Contents
ToggleHeterocyclic compounds are cyclic compounds with at least one atom other than carbon in the ring, like N, P, O, S, and so on. Most heterocyclic compounds contain only nitrogen, oxygen, and sulphur as heteroatoms. Five- or six-membered rings of heterocyclic compounds are the most stable. Thus, heterocyclic compounds may also be defined as five- or six-membered ring compounds with at least one heteroatom as the ring member. They are relatively stable and exhibit an aromatic character.
Examples of Heterocyclic Compounds
Heterocyclic components constitute more than half of all known organic compounds. They are extensively distributed in nature, and many of them play critical roles in life processes. For example;
- Nucleic acid bases containing purines and pyrimidines;
- Hemoglobin and chlorophyll containing porphyrin rings;
- Essential dietary ingredients containing vitamins B1, B2, B3, B6, and ascorbic acid;
- The three essential amino acids, namely, histidine, proline, and tryptophan;
- Nearly all drugs and pharmaceuticals; and
- Many natural products, such as alkaloids, carbohydrates, and plant pigments.
All of these chemicals have heterocycles (or rings) in their molecules.
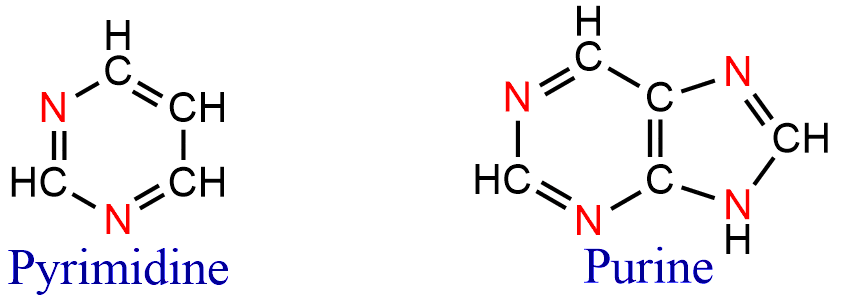
Classification of Heterocyclic Compound
There are primarily two types of heterocyclic molecules.
- Aliphatic heterocyclic compound
- Aromatic heterocyclic compound
Aromatic Heterocyclic compounds
Aromatic heterocyclic rings are compounds with structures similar to benzene but with a heteroatom substituting at least one carbon atom of the benzene ring or substituting more than one carbon atom with a heteroatom. Aromatic heterocycles are divided further into more categories;
Six-atom, six-π-electron aromatic heterocycles
Six-atom, six-π-electron aromatic heterocycles are pyridine, pyridazine, pyrimidine, pyrazine, 1,2,3-triazine, 1,2,4-triazine, 1,3,5-triazine, and 1,2,4,5-tetrazine.
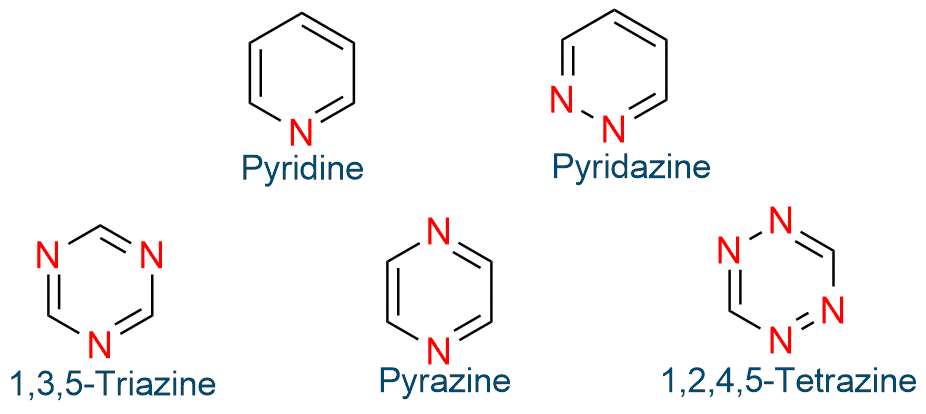
Five-atom, six-π-electron aromatic heterocycles
Five-atom, six-π-electron aromatic heterocycles are pyrrole, thiazole, thiophene, oxazole, isothiazole, 1H-pyrazole, 1H-imidazole, and 1H-tetrazole.
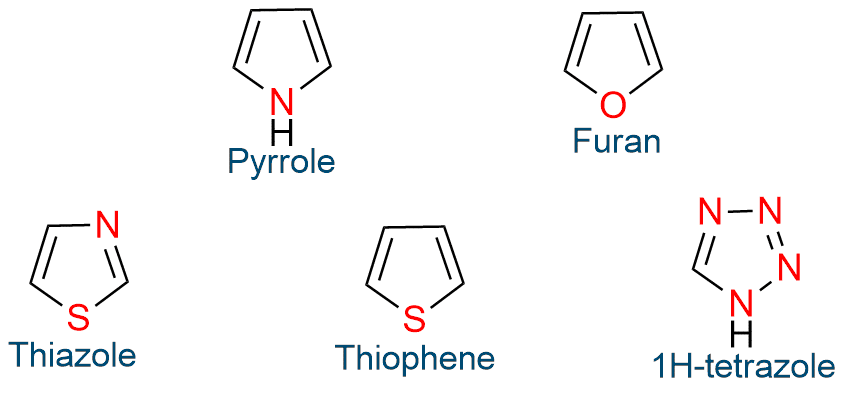
Fused ring system aromatic heterocycles
There are fused ring system aromatic heterocycles. For example, quinoline, isoquinoline, cinnoline, quinazoline, quinoxaline, phthalazine, indole, isoindole, and benzimidazole.
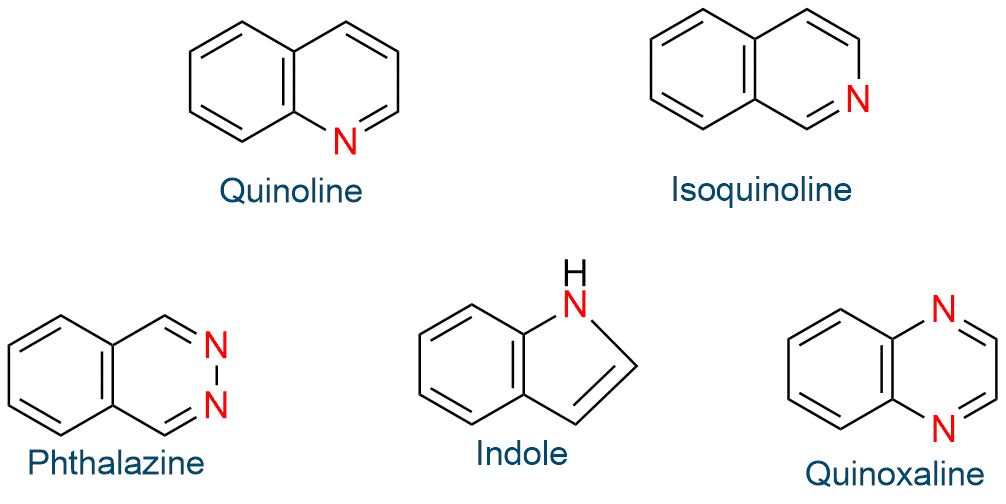
Non-aromatic Heterocyclic compounds
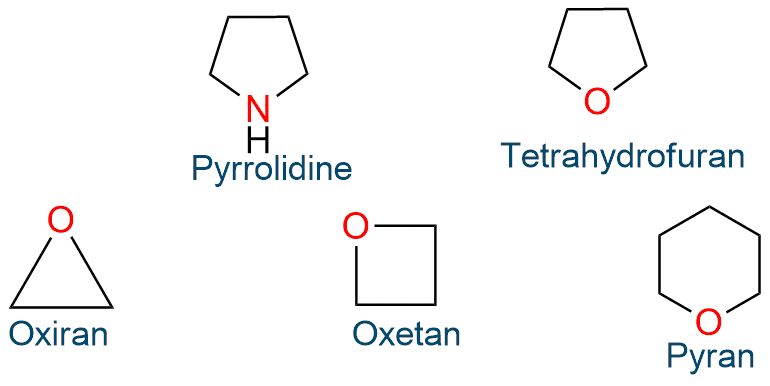
Nonaromatic small ring heterocycles that may be partially or fully saturated. There is no possibility of cyclic delocalization of p-electrons in these heterocyclic compounds, therefore they lack aromaticity, and these small-ring heterocycles suffer from significant angle strain. For example, pyrrolidine, tetrahydrofuran, thiolan, pyran, aziridine, oxiran, azitidine, oxetan, and so on.
Nomenclature of Heterocyclic Compound
Naming heterocyclic compound is challenging due to the availability of several common names in addition to the universally established systematic nomenclature. The name of a monocyclic compound is prefixed with the name of the heteroatom. For example,
- nitrogen→aza,
- sulfur→thia,
- oxygen→oxa,
- silicon→sila,
- phosphorous→phospha, and
- boron→bora.
The suffixing is determined by the nature of the heteroatom-nitrogen-containing heterocycles, and heterocycles without nitrogen are suffixed in numerous but distinct ways. Aromatic and nonaromatic heterocycles have distinct but similar suffixes.
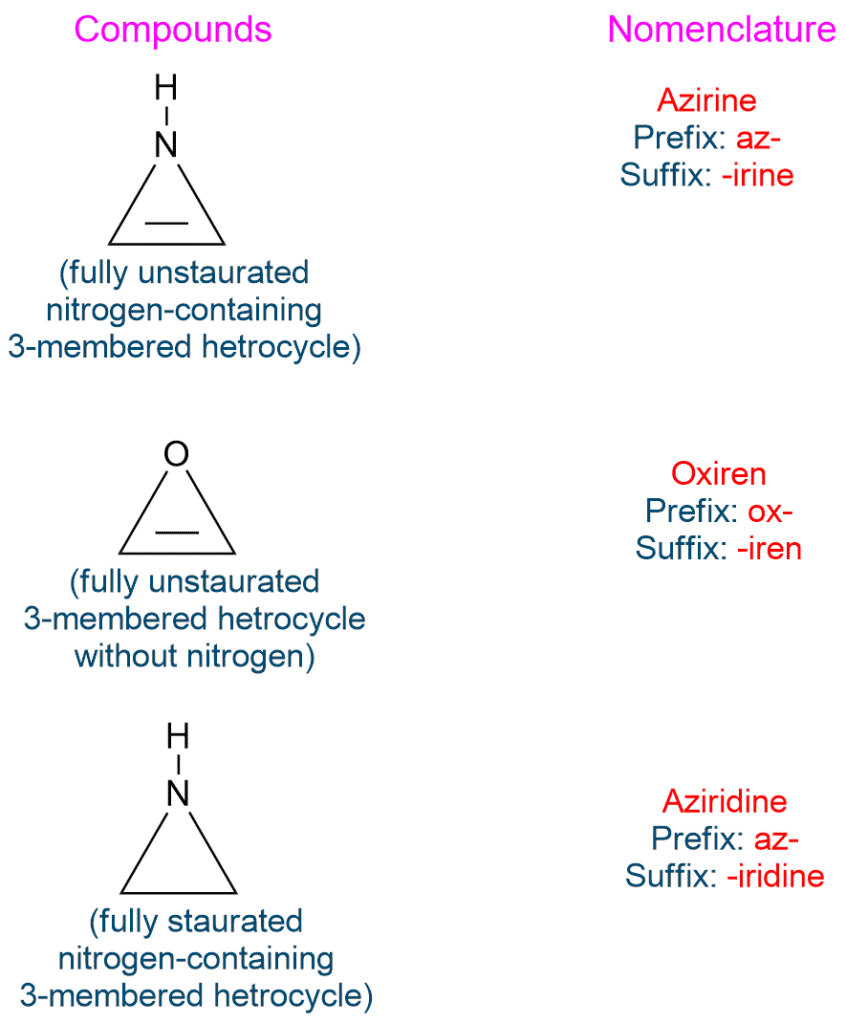
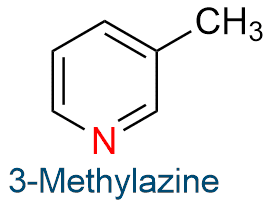
In a monocyclic molecule, numbering begins with the heteroatom and goes in the direction where the substituents get a lower location.
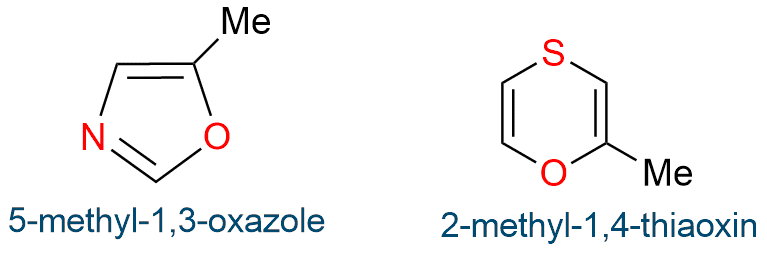
When there are many heteroatoms in a monocyclic molecule, the order of priority of the heteroatoms varies as follows.
- If the group number of the heteroatom is different, the atom of the higher group number gets a higher preference, for example, O (Gr. vi) > N (Gr.v).
- If the group number of heteroatoms are the same, then the lighter atom is preferred, for example, O (atomic mass 16) > S (atomic mass 32).
Uses of Heterocyclic Compound
- Heterocyclic compounds are employed in the agricultural and pharmaceutical sectors.
- These compounds are utilized as starting materials in the synthesis of organic molecules.
- Heterocyclic chemicals are employed in corrosion inhibitors, sanitizers, anti-ordinates, and developers.
- Pesticides, dyes, and plastics contain heterocyclic compounds.
- The heterocyclic compound azo dye shows anti-mycobacterial activity.
- Other heterocyclic compounds show anticancer activity, antiparasitic activity, and antioxidant activity.
FAQs
What are heterocyclic compounds?
Heterocyclic compound are those where one or more atoms of the ring are heteroatoms, for example, N, O, S, P, etc.
Are all heterocyclic compounds aromatic?
Not all the heterocycles are aromatic, there are other nonaromatic small ring heterocycles like Pyran, Thiolan, Oxetan, etc.
Is benzene a heterocyclic compound?
Because there are no heteroatoms in the ring, benzene is not a heterocyclic compound.






