Table of Contents
ToggleBefore explaining chemical shift reagents, let’s learn the major problem of the low-field spectrum(low resolution) and how these problems can be overcome.
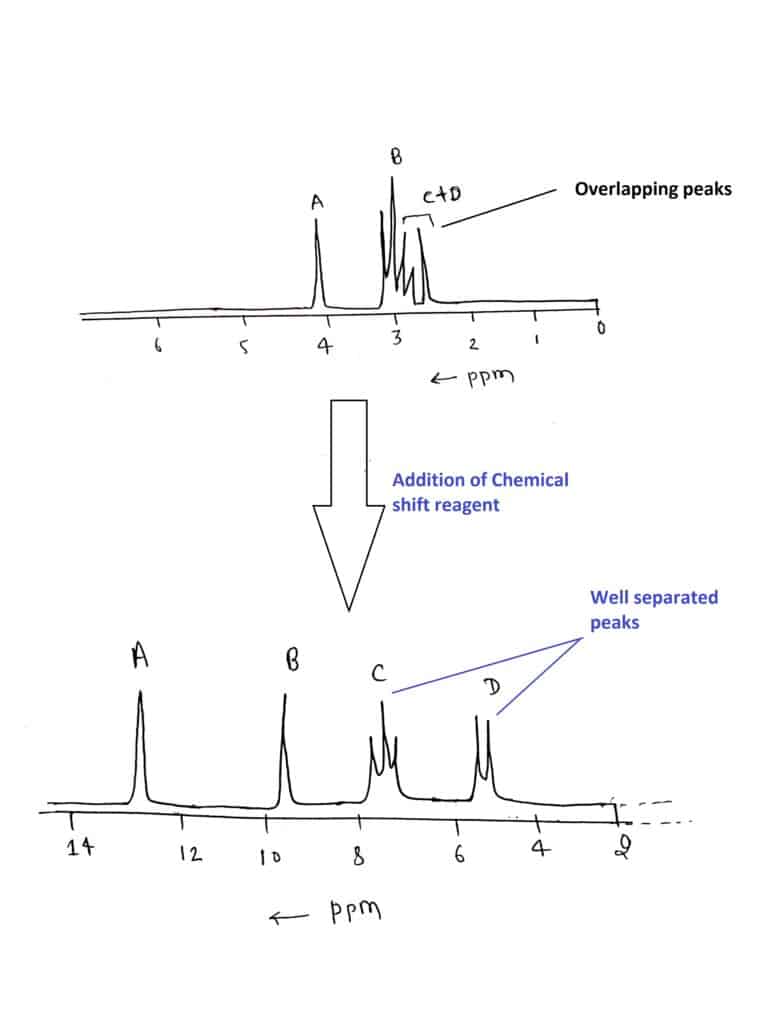
In the low field spectrum(60 or 90 MHz) of an organic compound, there is often peak overlap so extensively that individual peaks and splitting can not be extracted. This problem can be simplified by
- Using a spectrometer that operates at a higher frequency (300MHz or 500 MHz). At high field strengths, the coupling constant value doesn’t change but the chemical shifts in Hertz of the protons responsible for multiplets do increase.
- Selective spin decoupling(double resonance)
- Using chemical shift reagents(NMR shift reagents)
Chemical shift reagents
Chemical shift reagents are organic complexes of paramagnetic rare earth metals from the lanthanides series. The addition of these reagents to a compound containing an appropriate functional group spreads out the NMR absorption pattern due to profound shifts in the resonance positions of the various groups of protons.
Examples of Chemical shift reagents
Most of the commercially available shifts reagents are paramagnetic chelates of europium. Two of its widely used complexes are:
- Tris-(dipivalomethanato) europium [Eu(dpm)3]
- Tris-(6,6,7,7,8,8,8-heptafluoro-2,3-dimethyl-3,5-octanedionato) europium [ Eu(fod)3]
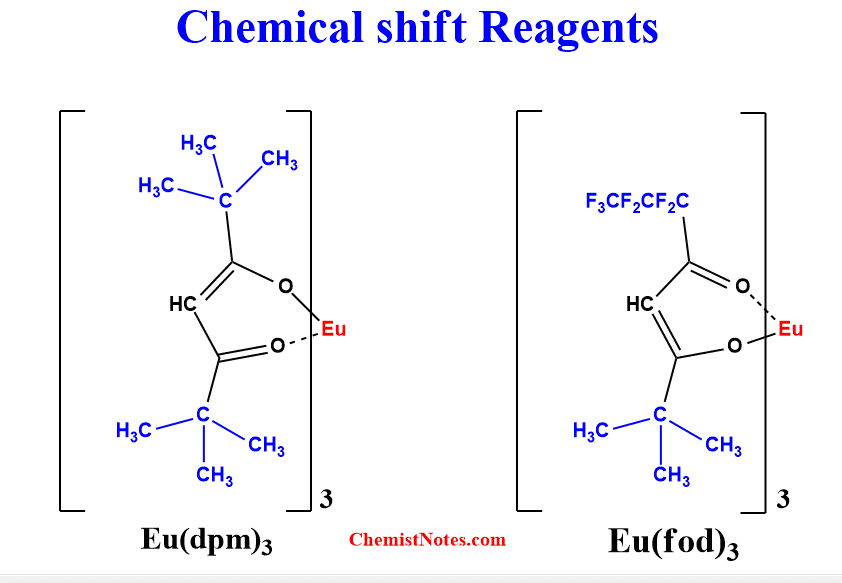
The directions of shifts depend primarily on which metal is used.
- Complexes of Europium, erbium, thulium, and ytterbium shift resonance to the lower field(larger chemical shift value).
- Complexes of Cerium terbium, praseodymium, neodymium, holmium, and samarium generally shift resonance to a higher field.
The extent of the shift of chemical shift value of protons induced by using shift reagents depends on the following factors.
- The distance between the metal (Eu3+) and groups of protons.
- The concentrations of the shift reagents in the solution.
How does chemical shift reagent work?
The chelates form a complex with the functional group of the substrate(compound) and induce spectral simplification in the NMR spectrum. These chelates are Lewis acid and the lone pair of electrons of the functional group coordinate with chelate(Eu3+). Therefore, aldehydes, ketones, alcohols, thiols, ethers, and amines all interact with these chelates.
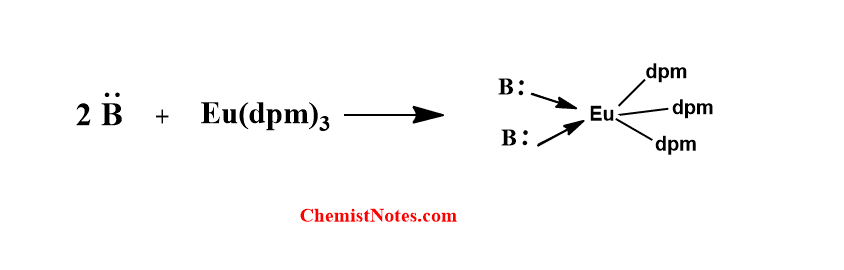
Advantage of Chemical shift regents
These reagents shift the value of chemical shifts similar to those observed at the higher field without the purchase of a high-field NMR instrument.
Limitations of shift regents
- The europium cations of the shift reagents cause a small amount of line broadening by decreasing the relaxation time of protons in the sample. At high shift reagent concentrations, this problem becomes serious.
- The hygroscopicity of the reagents and the susceptibility of the complexes to traces of moisture requires dry box techniques.
Chiral shift regents
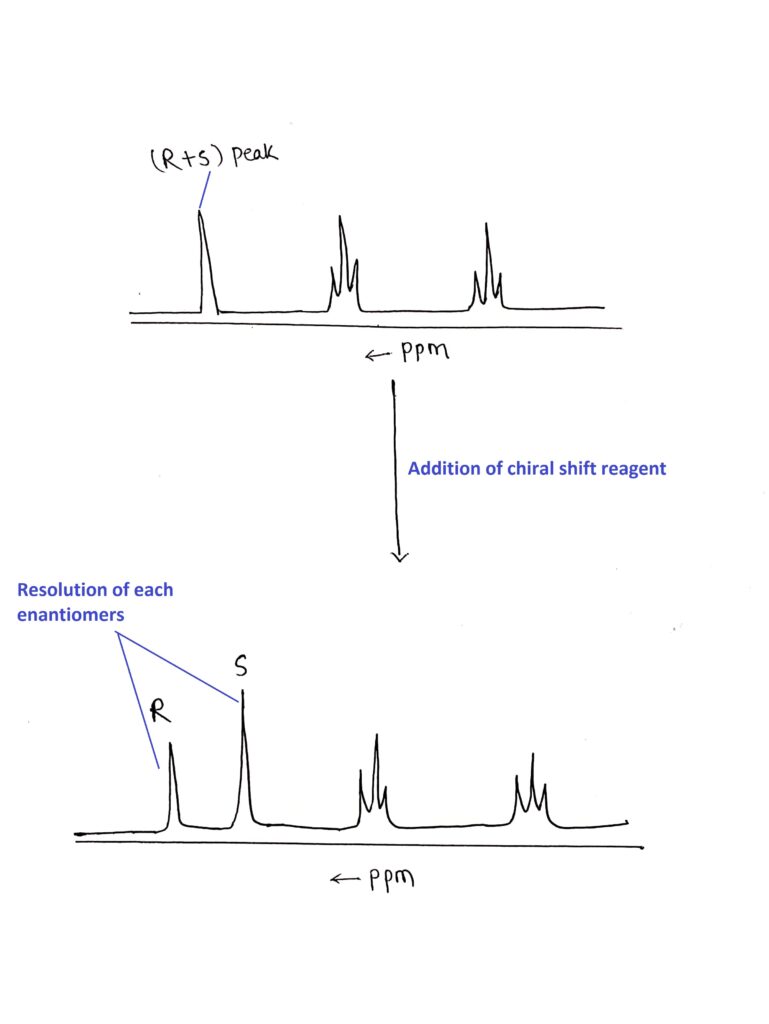
Lanthanide complexes in which the organic ligand on the metal is optically active create a chiral shift reagent. These are often known as chiral lanthanide shift reagents. One of the commonly used such reagents is tris-[3-(heptafluoropropyl hydroxyl methylene)-d-camphorato] europium (III) [Eu(hfc)3]. During structural elucidation, chiral shift reagents are used to simplify the spectrum. Moreover, these can be used to determine the proportion of R/S forms present in the enantiomeric mixture.
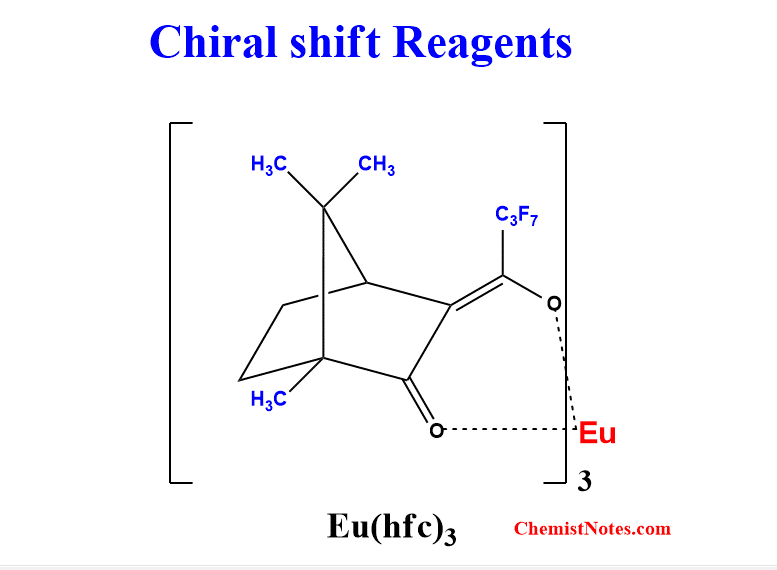
When Eu(hfc)3 complexes to chiral molecules(enantiomers identical protons), diastereomeric complexes are formed. These diastereomeric complexes give rise to different chemical shifts for protons that are then identified.
The use of chemical shifts reagents requires a functional group that is a strong enough base to form a stable complex, a sufficient resolution to separate the corresponding peaks in each diastereomeric complex, and freedom from interfering peaks.
How does Chiral lanthanide shift reagent separate enantiomers?
As we know, enantiomers have protons having the same chemical shift value which makes it difficult to detect which one is R form and which is S form. However, two enantiomers under investigation can have a different binding affinity with the chiral shift reagents. Moreover, these enantiomers can adopt different conformations on binding with shift reagents. This makes different chemical environments for the protons in these formed diastereomers. Therefore, the chemical shift values are shifted to different extents, and enantiomers separate.
FAQs/MCQs:
What is the full form of Eu(dpm)3?
The full form of Eu(dpm)3 is Tris-(dipivalomethanato) europium.
What is the full form of Eu(fod)3?
The full form of Eu(fod)3 is Tris-(6,6,7,7,8,8,8-heptafluoro-2,3-dimethyl-3,5-octanedionato) europium.
What is the full form of Eu(hfc)3?
The full form of Eu(hfc)3 is tris-[3-(heptafluoropropyl hydroxyl methylene)-d-camphorato] europium (III).






