Table of Contents
ToggleConformation and Configuration are the two important part of stereochemistry that deals with the study of molecular symmetry or molecular geometry or simply how the atoms or group are arranged in the structure. The terms “conformation” and “configuration” both refer to a certain molecule’s spatial arrangement. These terms are mostly used in organic chemistry to identify the spatial arrangement of atoms in organic molecules.
Conformation
Conformation or conformers are obtained by the rotation of a single bond between carbon and carbon. Those that cannot be separated at room temperature because they interconvert quickly. Rotation about C-C single bonds yields them (and from inversion of the electron pair on nitrogen). They are also called rotational conformation.
Different spatial arrangements of carbon atoms in space are seen as a result of this rotation, and these spatial arrangements can convert into one another. The term “conformation,” “conformer,” or “rotamer” refers to this spatial arrangement of carbon and hydrogen atoms that can be transformed into one another by rotation around a C-C single bond. As a result, by rotating around C-C single bonds, alkanes can adopt an infinite variety of conformations. Due to repulsive interactions between the electron clouds of C-H bonds, this rotation is not completely free. The torsional strain seems to be the name given to this opposing interaction.
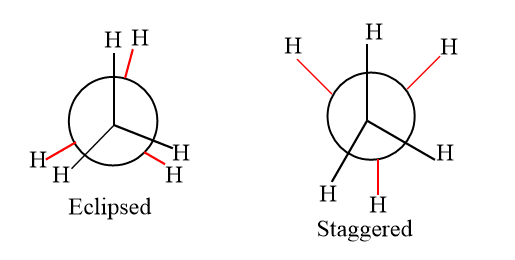
The resulting isomers cannot be isolated if there is little barrier to rotations about a single bond. The isomers rapidly interconvert. The isomers can be separated, though, if the barrier is sufficiently strong to prevent their quick interconversion. For instance, even in a simple molecule like ethane, where the staggered conformation is more stable than the eclipsed conformation, there are barriers to rotation. Conformation can be explained in terms of Newman and sawhorse projection. They are broadly divided into two types.
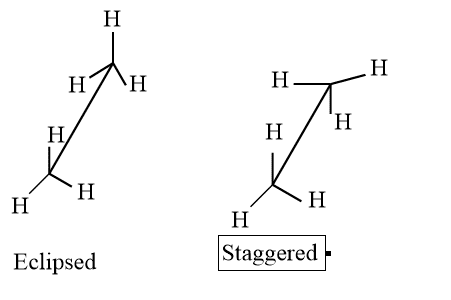
- Eclipsed: The conformation in which hydrogen atoms are attached to two carbon areas nearest to each other as possible is called eclipsed conformation.
- Staggered: The conformation in which hydrogen atoms attached to two carbons are as far as possible with respect to each other is called staggered conformation.
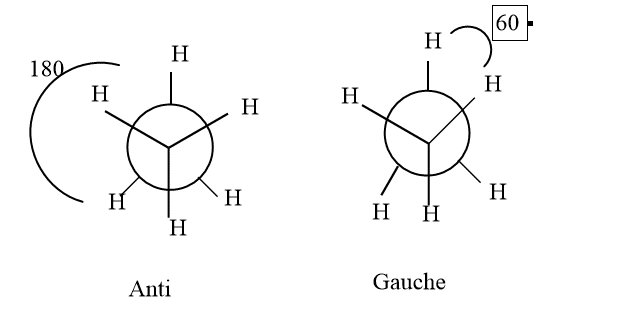
The staggered conformation of ethane has two relationships based on their rotation of 180 degrees and 60 degrees.
Configuration
The spatial arrangement of atoms or groups at the carbon atom is called configuration. It is permanent geometry and tells about how the atoms or molecules were connected. Examples L and D, R and S configuration. They are classified as relative and absolute configurations.
- Relative configuration: In the relative configuration, the relation of the configuration of one molecule to another is established.
- Absolute configuration: When the configuration of a given stereoisomer is known, its absolute configuration is said to be known.
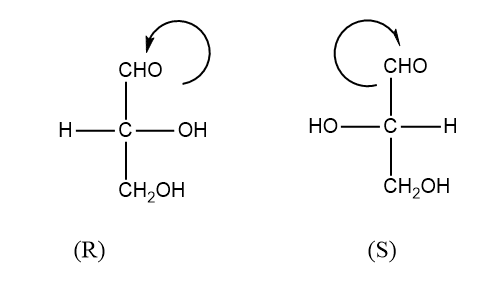
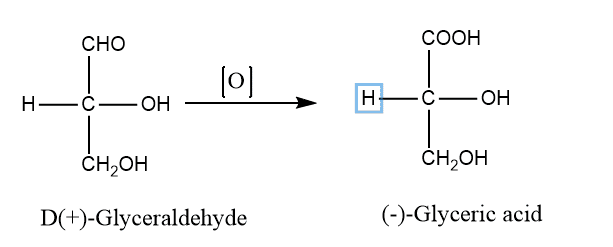
Note: D and L refer to stereochemical relation but d and l refer to the actual direction of rotation.
We can determine the configuration of geometrical isomers by various physical and chemical methods which you can easily read in our recently written article. You can check that out too.
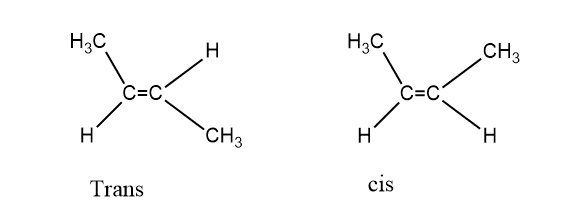
Difference between conformation and configuration
The difference between conformation and configuration is shown below:
| Conformation | Configuration |
| It is the arrangement of atoms or groups which are interconvertible by rotation around a single bond. | It is the spatial arrangement of atoms or groups at the carbon atom. |
| Interconversion does not need the making and breaking of bonds. | Possible only through breaking and making of bonds. |
| They cannot be isolated from each other. | They can be isolated from each other. |
| Leads to conformational isomerism. | Leads to geometrical and optical isomerism. |
| example conformation of ethane | absolute and relative configuration |
MCQs/FAQs
What are conformations?
The different spatial arrangement of atoms or molecules that arises by the means of rotation about a c-c single bond.
What is configuration?
The relative position of atoms or molecules can be changed by cleaving and forming new bonds.
References
- K. R. Palak, Stereochemistry, Pairavi Prakashan, Kathmandu, Nepal, 2017.






