Table of Contents
ToggleIn this article, we have tried to explain the NMR spectroscopy principle, instrumentation, chemical shifts value, factor affecting its value, and application of this technique in organic chemistry in simple language. Let’s start.
NMR spectroscopy (Nuclear magnetic resonance spectroscopy) is a nondestructive analytical technique to identify carbon, and hydrogen frameworks within the molecules. In other words, the study of the absorption of radiofrequency radiation by nuclei in a magnetic field is called nuclear magnetic resonance(NMR). Typically, there are two kinds of Nuclear magnetic resonance techniques.
- 1H-Nmr
- 13C-Nmr
Basic Principle of NMR spectroscopy
NMR involves the study of the interaction between electromagnetic radiation in radiofrequency and the nuclei of atoms. A nucleus with an odd number of protons or an odd number of neutrons such as 1H,13C,15N,19F possesses nuclear spin and such nuclei can only be examined by NMR.
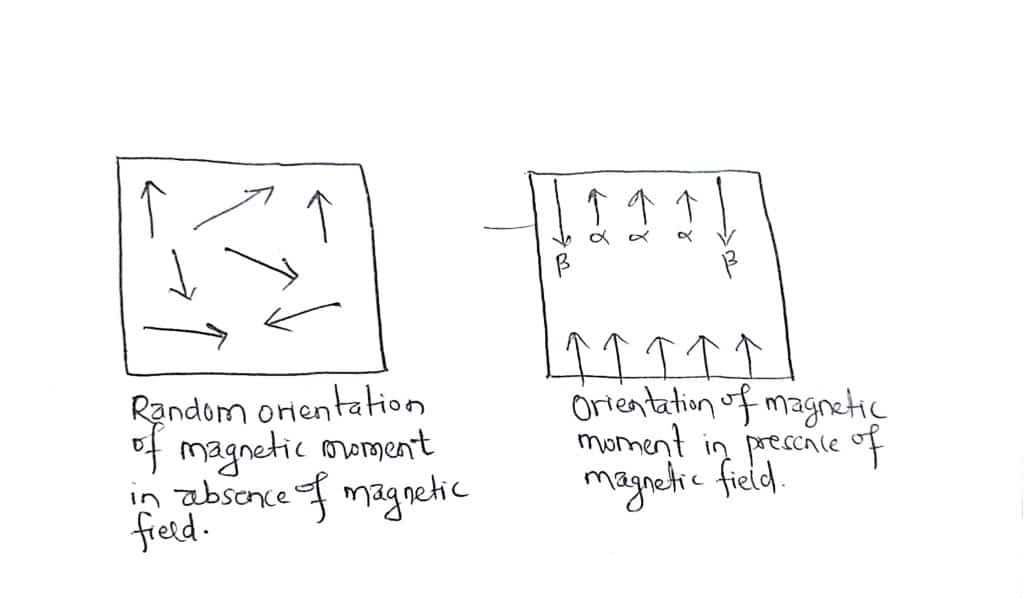
If a charged particle-like proton/nucleus spins about its own axis, it behaves like a tiny magnet and it generates a magnetic field called magnetic moment. Without an external magnetic field, the nuclear spins are in random directions. When the nucleus of an atom is placed in an external magnetic field, the interaction between the magnetic moment takes place. The magnetic moment must align either with the external field or against the field.
If a nucleus is aligned with the magnetic field, it is said to occupy the α-spin state and if it is aligned against the field, it is said to occupy the β-spin state. The two spin states are not equivalent in energy, there is a quantifiable difference in energy between them.
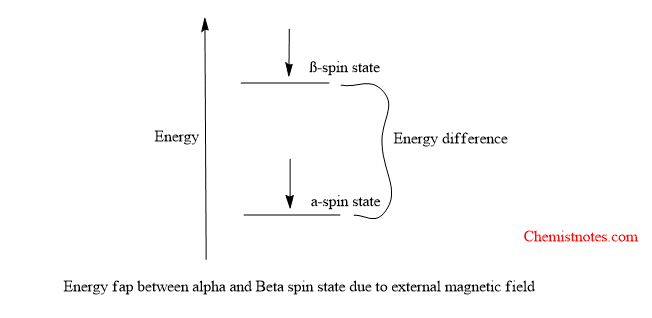
The external magnetic field establishes an energy gap between spin states. The magnitude of the energy gap depends on the strength of the external magnetic field. The energy gap increases with increasing magnetic field strength.
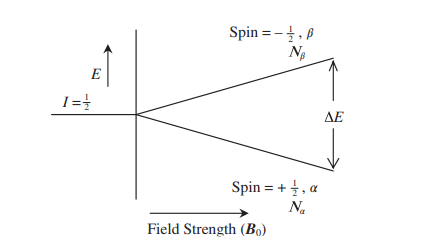
When the energy between the two energy states lies within the ranges of the radiofrequency radiation then the α-spin state absorbs energy and flips to the β-spin state and the nucleus is said to be in nuclear magnetic resonance.
In the presence of an external magnetic field, the electron density around the nucleus circulates which produce a local magnetic field that opposes the external magnetic field. This effect is called diamagnetic. Due to the induced magnetic field established by the circulating electron density, the nuclei/proton experiences a slightly smaller net magnetic field than an actual magnetic field. This effect is called the shielding effect.
Some nuclei/protons are surrounded by more electron density and are more shielded while others are surrounded by less electron density and are less shielded or deshielded. Therefore, protons/nuclei in the different chemical environments have different energy gaps between the α-spin state and the β-spin state and hence, absorb different frequencies of radiofrequency radiation.
NMR spectroscopy graph
A plot of the frequencies of the absorption peaks versus peak intensities constitutes an NMR spectrum. The signals on the left side of the spectrum(downfield) are high-frequency signals. Signals on the right side of the spectrum(upfield) are low frequencies signals because they result from shielded protons that absorb lower frequencies of radiation.
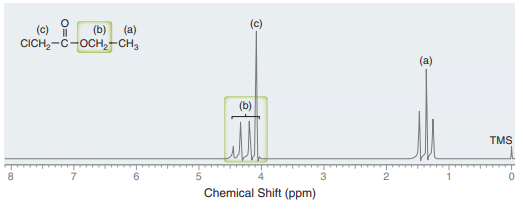
NMR spectroscopy table
The NMR spectroscopy table is shown below:
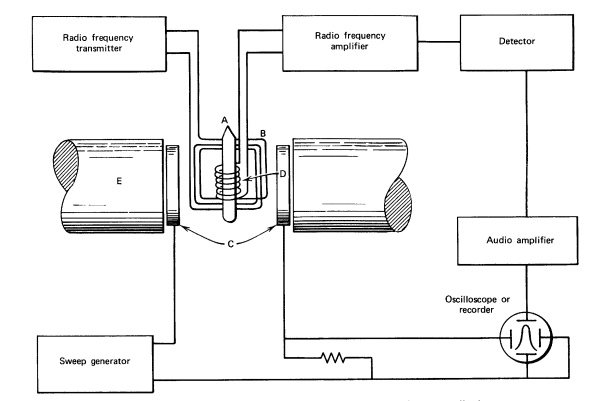
Instrumentation/Diagram of NMR spectroscopy
The instrumentation of NMR spectroscopy is shown below:
Uses/application of NMR Spectroscopy
The major application of NMR spectroscopy are listed below:
- Identification of functional groups: Every functional group has a characteristic signal in the NMR spectrum. By studying the chemical shift of the compound, it is possible to identify the functional group in the compound.
- Structure elucidation of unknown compound: By observing the chemical shift values and splitting of the signal of protons under different environments, it is possible to place hydrogen at a suitable place in the formula and hence the structure of a given compound can be established.
- Comparison of two compounds and determination of the molecular weight: By comparing the peak of the unknown compound with the of an unknown compound, the molecular weight can be determined.
- Distinction between cis-trans isomer and conformers: Protons have different values of chemical as well as coupling constants in cis and trans isomer. Therefore, it is easy to identify cis and trans isomers.
Factors affecting NMR spectroscopy
Some of the factors that affect NMR Spectroscopy are given below:
- Nature of group
- Electronegativity value
- Inductive effect
- Hybridization type
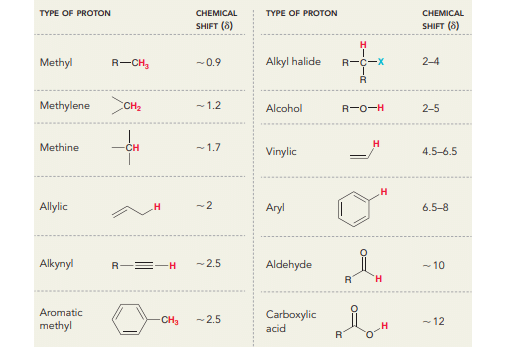
Chemical shift in NMR spectroscopy
Chemical shift is defined as the difference in parts per million(ppm) between the resonance frequency of the observed proton and that of the reference compound, tetramethylsilane(TMS).

Factors affecting chemical shift values in proton NMR spectroscopy are listed below:
- Nature of group: If the electronegative atoms like halogens, oxygens, and nitrogens are linked with protons they deshield the protons resulting in downfield absorption. Electron-releasing groups increase the electron density around the protons and result in upfield absorption.
- Hybridization: Hybridization affects the value of chemical shifts. Sp2 carbon is more downfield than Sp3 carbon.
Coupling constant(spin-spin splitting in NMR spectroscopy)
The splitting of a signal occurs due to the effects of neighboring protons. The neighboring proton with a different chemical shift can influence the signal of the other. If the protons are in the same chemical environment, such protons are said to be equivalent and absorb the same magnetic field strength and they behave as one proton.
If the protons are in different chemical environments, such protons are said to be non-equivalent protons and absorb different magnetic field strengths. Therefore, the splitting of the peak takes place. When signal splitting occurs the distance between the individual splitter peak is always constant which is called the coupling constant(J). It is measured in hertz.
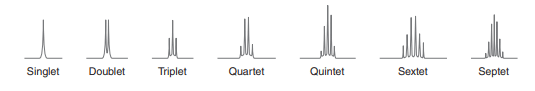
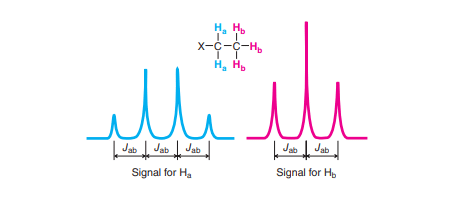
Integration in NMR spectroscopy
In NMR spectroscopy, the process of measuring the area of an NMR signal is called Integration. The value of integration indicates the number of protons giving rise to the signal.
Computers automatically measure the area of each signal and generate integration values. The values only have meaning when compared to each other and give a ratio. Using this ratio, the number of protons present in the signal can be determined.
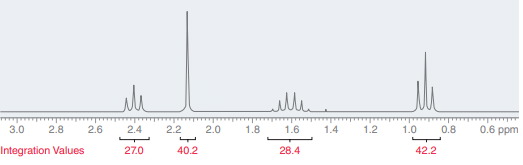
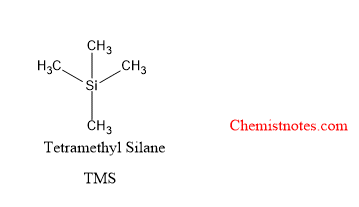
Why tms is used as reference compound in NMR spectroscopy?
Tetramethylsilane is a universally accepted reference compound because of the following facts.
- TMS is chemically inert, symmetrical, volatile, and soluble in most organic solvents.
- TMS gives a signal, intense, sharp NMR peak, and its protons are more shielded than almost all other protons in the organic compounds.
noesy nmr spectroscopy
The nuclear Overhauser effect (NOE) results from a type of nuclear spin relaxation, involving pairs of spins, called cross-relaxation. One of the useful applications of the NOE is, therefore, to determine which protons are close in space to which other protons. The NOE can be observed by irradiating a peak corresponding to a particular proton in the sample with lower power radiation than is used for decoupling.
tocsy nmr spectroscopy
Total correlation spectroscopy shows the entire chain of protons in the molecule, each of which is coupled to next.
NMR spectroscopy youtube
NMR spectroscopy video
FAQs/MCQs:
what is NMR spectroscopy?
NMR spectroscopy (Nuclear magnetic resonance spectroscopy) is a nondestructive analytical technique to identify carbon, hydrogen framework within the molecules.
why tms is used as reference in nmr spectroscopy?
TMS is used as a reference in NMR spectroscopy because TMS gives a signal, intense, sharp NMR peak and its protons are more shielded than almost all other protons in the organic compounds.







2 Responses
I loved the way NMR SPECTROSCOPY is explained. The language is so simple and to the point.
Yet , I would be more pleased if you could add the *working part* of NMR SPECTROSCOPY in this notes.
Thankyou so much.
Hi Pratibha mam, thank you very much for your appreciation. I will update soon.