Table of Contents
ToggleEnantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other. The molecule is non-superimposable only if the pure compound is optically active. Any materials that rotate the plane of polarized light are optically active compounds, and the property of nonsuperimposability of an object on its mirror image is called chirality. In general words, Enantiomers are the optical isomers of chiral molecules that are non-superimposable mirror images of each other. Enantiomers are also known as enantiomorphs.
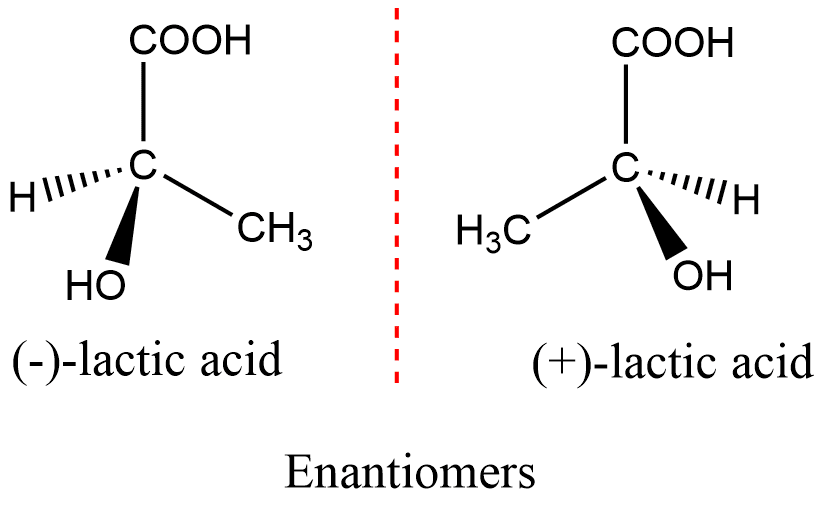
A mixture of an equal amount of enantiomers is called racemic mixtures. Such mixtures are optically inactive since the equal and opposite rotation cancels.
Criteria for Enantiomers
The essential criteria are listed below.
- Isomers should be optically active.
- Chirality is required.
- Nonsuperimposable mirror images of each other.
Properties of Enantiomers
Enantiomers exhibit the same physical and chemical properties except in the following points:
- They rotate the plane-polarized light in the opposite direction, but in equal amounts. The isomers that rotate plane-polarized light in a clockwise direction are known as dextro (+) isomers, while those which rotate in opposite directions are known as levo (-) isomers/enantiomers.
- When enantiomers react with optically inactive reagents, their reaction rates are identical, but this is not the case with optically active compounds. When two enantiomers are treated separately with the same optically active reagent, the two transition states obtained are not identical. It demonstrates that they have different activation energies. As a result, it’s clear that the two enantiomers react at different speeds with the optically active reagent.
- In asymmetric environments, enantiomers have identical properties, but in an unsymmetrical environment, they may have different properties.
- When enantiomers react with inactive molecules in the presence of an optically active catalyst, their reactivity may differ.
- Although pure compounds that consist of chiral carbon are optically active, mixtures of equal amounts of enantiomers are optically inactive due to equal and opposite rotations.
Enantiomers vs Diastereomers
The major differences between enantiomer and diastereomer are tabulated below.
| Enantiomers | Diastereomers |
| Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other. | Diastereomers are stereoisomers that are formed by the same molecular formula but are not mirror images of each other. Learn more. |
| Enantiomers are Optically active. | May or may not be optically active |
| Possesses the same physical and chemical properties except for the rotation of plane-polarized light. | Possesses different physical and chemical properties. |
| Have only one stereocenter. | Have more than one stereocenter. |
| The shape of the isomers/molecules are similar. | The shapes of the molecules are different. |
| Present in pairs. | May be in several molecules. |
| Separation of enantiomers is difficult. | Separation can be achieved by chromatography, fractional crystallization, fractional distillation technique, etc. |
Enantiomers video
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






